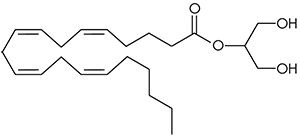
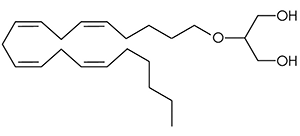
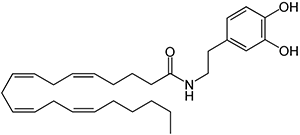
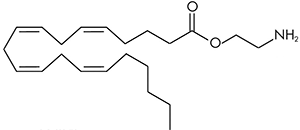
There are a huge variety of synthetic cannabinoids possible, including variations on THC and other natural cannabinoids, the CP series created by Pfizer in the 1970s but never brought to market, anti-Alzheimer’s treatment HU-210, and aminoalkylindole derivatives like WIN 55,212-2. But the most popular and prevalent synthetic cannabinoids in today’s recreational drug market are a subset of what are colloquially known as “JWHs”, after the researcher who completed a large body of work on them, John W. Huffman. There are several hundred of these compounds, named in the format JWH-001 and up. Some of the first compounds in this long list to be diverted to the recreational drug market are certain alkylated naphthoylindoles, which also happen to be very easy to synthesize. Have you used “herbal incense”, “Spice”, “K2”, or any of the other cannabis substitutes that have been popping up lately? A safe bet is that they’re an inactive herbal carrier with these synthetic cannabinoids dissolved in a solvent sprayed on top.

Synthetic cannabinoids sold in this manner drive a large and profitable industry, a strange bastard child of prohibition that would simply not exist if the sale of cannabis was regulated. While a small subset of informed drug users would likely still create demand for pure versions of these products, “incense” consumption would be effectively zero if the general population had access to quality cannabis and did not have to worry about metabolic products being tested for.
Imagine the state of the synthetic cannabis industry a few years ago. There have been whispers of some interesting cannabinoids being developed by academics for medical use and research. But how is it possible to tell which of these might substitute for THC in recreational users? Let’s investigate the binding affinity, a measure of how well a compound binds to a receptor. We can use Ki values to do this, which measure how concentrated the compound must be in order for it to have an equal chance of being bound to a receptor or not. Lower values mean high potency as only a small amount of compound is required, while higher values indicate lower potency. While having similar binding affinities doesn’t always mean similar recreational effects, it can certainly help narrow the field. Let’s see how the first seven alkylated naphthoylindoles stack up against THC.
| Compound |
CB1 Ki (nM) |
CB2 Ki (nM) |
Image |
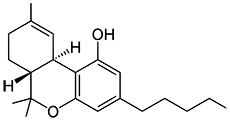
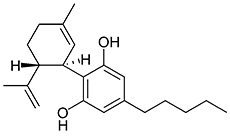
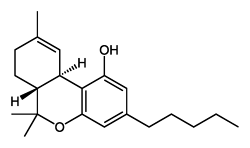
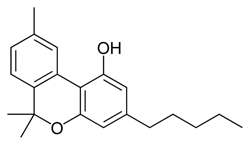
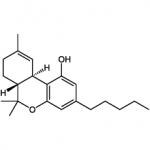
THC
(reference) |
40.7 ± 1.7 |
36.4 ± 10 |
 |
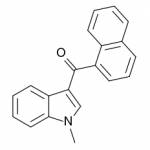
JWH-070
(methyl) |
>10000 |
>10000 |
 |
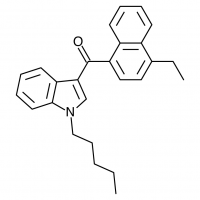
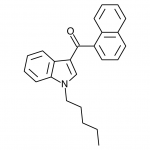
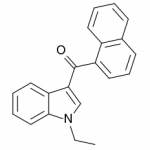
JWH-071
(ethyl) |
1340 ± 123 |
2940 ± 852 |
 |
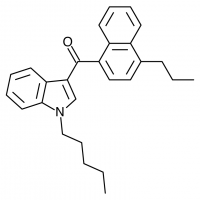
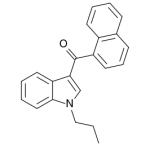
JWH-072
(n-propyl) |
1050 ± 55.0 |
170 ± 54.0 |
 |
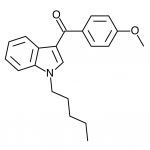
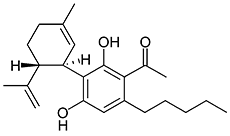
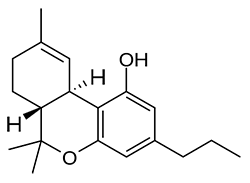
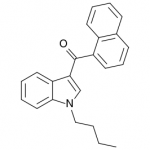
JWH-073
(n-butyl) |
8.90 ± 1.80 |
38.0 ± 24.0 |
 |
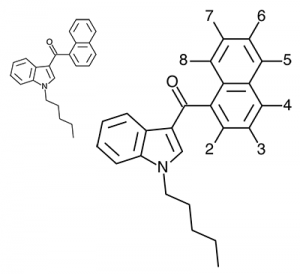
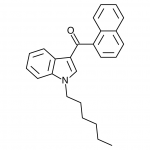
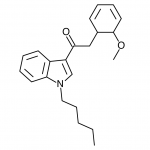
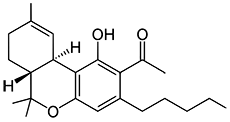
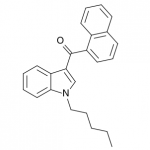
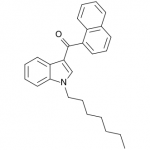
JWH-018
(n-pentyl) |
9.00 ± 5.00 |
2.94 ± 2.65 |
 |
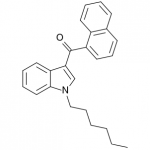
JWH-019
(n-hexyl) |
9.80 ± 2.00 |
5.55 ± 2.00 |
 |
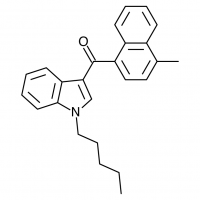
JWH-020
(n-heptyl) |
128 ± 17.0 |
205 ± 20 |
 |
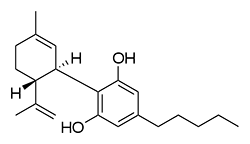
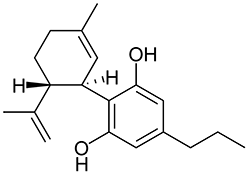
The shorter alkyl chain lengths of JWH-070 (ethyl) and JWH-071 (methyl) show negligible activity. As we move to the propyl chain of JWH-072 we see little change in CB1 binding affinity but CB2 affinity increases almost 15 times. JWH-073 and its butyl chain are a bit more promising, with higher affinity for CB1 and a similar affinity for CB2 compared to THC. JWH-018 (pentyl) and JWH-019 (hexyl) stand out, with higher potency at both receptors than THC itself. So far so good, but as we increase the chain length in JWH-020 (heptyl) our streak ends, as we see a a 13 fold decrease in binding affinity at the CB1 receptor and a 40 fold reduction at the CB2 receptor.
Activity appears to peak around the five carbon pentyl chain in these naphthoylindoles, as opposed to the classical cannabinoids which peak at the seven carbon heptyl chain. Judging from binding affinities It looks like JWH-073, JWH-018, and JWH-019 would be the best bets - so did any of them pan out?
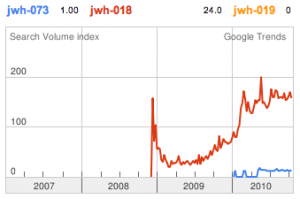
JWH-018 is likely the world’s most popular synthetic cannabinoid, and was one of the first introduced for wide sale. It entered the public psyche in late 2008 when German company THC-Pharm identified it as a primary ingredient in the “Spice” herbal smoke blend. Quickly outlawed in Germany and several other European countries in early 2009, it has since gained popularity in other markets including the US. Active in smoked dosages of only a few milligrams, tolerance builds quickly revealing a dirty secret relative to natural cannabis. JWH-018 is a single compound active as a full cannabinoid agonist, while cannabis acts as a mixture of many phytocannabinoids including partial agonists and antagonists. This complex balancing act provides a safety net that JWH-018 lacks, as the synthetic compound can activate cannabinoid receptors in a far more specific and potent manner. For instance, GABA may be inhibited far more effectively than natural cannabis, leading to severe anxiety and reduced seizure threshold at high doses. This may be the origin of the infamous “fear” JWH-018 is known to induce in overdose.
JWH-073 is not as potent as JWH-018, but has arguably a more pleasant high with less anxious effects. After JWH-018 was outlawed in Germany, a seizure and analysis of a new batch of Spice shipped only 4 weeks after the ban showed that the manufacturers had quickly switched active ingredients to the uncontrolled JWH-073.
JWH-019 has not reached the same fame as the others likely due to timing and distribution rather than any real shortcomings. It was introduced to a more saturated market along with many other competing synthetic cannabinoids after JWH-018 and JWH-073 were banned in some areas. This is likely to represent the new normal, as a wide number of these compounds targeting various recreational effects pop in and out of the market depending on economic and legal factors.
Mie Mie Aung, Graeme Griffin, John W Huffman, Ming-Jung Wu, Cheryl Keel, Bin Yang, Vincent M Showalter, Mary E Abood, Billy R Martin, Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding, Drug and Alcohol Dependence, Volume 60, Issue 2, 1 August 2000, Pages 133-140, ISSN 0376-8716, DOI: 10.1016/S0376-8716(99)00152-0.
 It’s an effect that many psychedelic users are familiar with - at the tail end of trip, with the experience waning, smoking cannabis will tend to increase the psychedelic effects and “bring the trip back”. Similar effects occur on the comedown of drugs like MDMA. Why does this happen?
It’s an effect that many psychedelic users are familiar with - at the tail end of trip, with the experience waning, smoking cannabis will tend to increase the psychedelic effects and “bring the trip back”. Similar effects occur on the comedown of drugs like MDMA. Why does this happen?