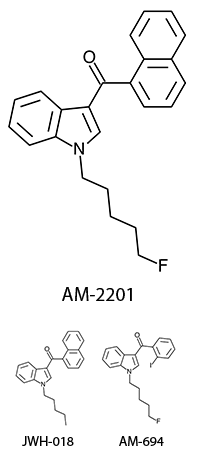
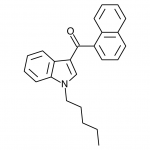
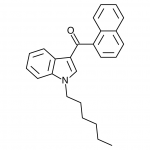
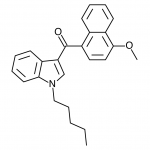
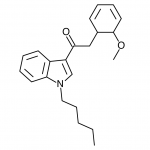
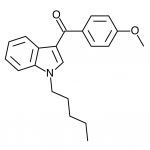
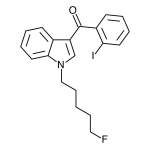
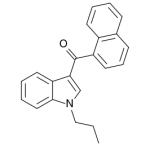
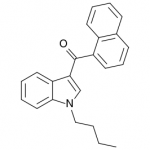
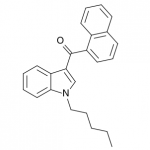
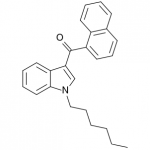
AM-2201 – A Hyperpotent Halogenated Unintended Consequence
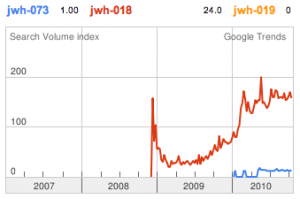
With the recent legal issues surrounding certain synthetic cannabinoids in the United States, the market has changed. Instead of economic pressures selecting certain cheap to produce cannabinoids with desirable effects, legal pressures have now created a situation where the inelastic demand of recreational drug users causes overseas manufacturers to seek the highest potency compounds in order to minimize risk of seizure. People still want to buy it, but they want to minimize the number of transactions, so send the tiniest package with the largest value.
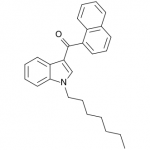
Previously in the market we saw various simple substitutions of the cheap, easy to synthesize, and potent original, JWH-018. But halogenated analogs, previously cast to the side due to more involved synthesis routes, are now becoming more popular due to the trend of increasing potency and duration. The difficulty in handling these very powerful compounds has caused them to be most prevalent in the commercial “smoke blends” market, where a fraction of these halogenated analogs can replace a far larger volume of the earlier generation cannabinoids. With these new legal pressures on end users however, we are seeing an increasing tendency toward the retail sale of these pure compounds.
The first of the halogenated AM series cannabinoids to reach the market was AM-694, discussed in Synthetic Cannabinoids: JWH-018 Replacements. This shares a fluoropentyl chain with AM-2201, and initially health concerns were raised about possible metabolism to toxic fluoroacetate. Luckily it appears that the odd-numbered (pentyl) chain means that the less toxic 3-fluoropropanoic acid will likely be produced instead, but this raises questions - if an even numbered fluoroalkylated chain was found to obscenely potent, would these health concerns prevent it from being brought to market? Or would the economic incentives of this crudely regulated market result in a product being sold with the excuse that only chronic long-term exposure is likely to result in apparent health risks? One would hope that manufacturers would not be so greedy, but history is not reassuring.
The effects of AM-2201 also appear to differ from natural cannabis and the first generation synthetic cannabinoids, both to start and as tolerance builds. Initially the effects are quite similar, although doses for AM-2201 are approximately a third of JWH-018. This has resulted in many reports of self-reported “seasoned” synthetic cannabinoid users having anxiety reactions as a result of apparent overdose due to increased sensitivity to inaccurate measurement. Tolerance builds quickly, and frequent users have reported psychedelic-style effects typically previously only associated with high-dose oral consumption of marijuana. The major difficulty with these high potency compounds is finding the “window” - that range of dosage where effects are strong enough to be considered enjoyable, but not so strong that peripheral effects such as anxiety or or dissociation affect the recreational potential. Measurement noise appears to translate to a rather inconsistently enjoyable experience.
It is frustrating that regulators appear to not consider the consequences of the time-tested inelastic demand of recreational drug users. Natural cannabis has been used for thousands of years with little ill effect, and its prohibition resulted in the birth of the synthetic cannabinoid market, compounds that have been known to science for at most a few decades. Now unthinking attempts to stamp out the first generation synthetic cannabinoid market have resulted in the promotion of hyperpotent substitutes patented in the mid-2000s. It is the very definition of unintended consequence to consider that regulatory promotion of these relatively unknown compounds could result in more public health concerns rather than less.