Phytocannabinoids
Cannabinoids are a class of compounds which display activity at the cannabinoid receptor, and are named after the cannabis plant from which they were first isolated. There are at least two types of cannabinoid receptors. CB1 receptors are found mostly in the brain, but are not present in the medulla oblongata, a part of the brain stem responsible for respiratory and cardiovascular functions. This demonstrates a major safety feature of cannabis, that recreational use does not present a significant risk of respiratory or cardiovascular failure. CB2 receptors are expressed primarily in the immune system, and appear to be responsible for anti-inflammatory and anti-convulsive effects.

Phytocannabinoids are naturally occuring cannabinoids found in the cannabis plant. They are concentrated in a sticky resin secreted by trichomes, hairlike structures covering the plant and concentrated in the flowering buds where the plant’s precious seeds are located. Not unlike our own body hair, it provides a barrier for the plant against marauding insects and other parasites. The sticky resin also reduces the rate of water loss, similar to the waxy outer layer of a cactus. There are a handful of easily isolated phytocannabinoids which have received the most study, although many more are present in smaller quantities.
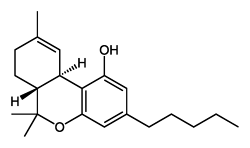
Tetrahydrocannabinol (THC) is the most well known phytocannabinoid of all, responsible for the majority of the recreational effects of cannabis when smoked. It is also more resistant to ultraviolet radiation than the other major phytocannabinoid CBD. Cannabis sativa is known for a higher ratio of THC to CBD and originates from hotter climates, which could be explained as a result of selection pressure from increased ultraviolet radiation in these equatorial regions. THC itself and sativas in general are known for a powerful, clean, and motivated high, but with possible anxiety occurring in higher dose ranges.
Cannabidiol (CBD) does not have the name brand recognition of THC, but can represent up to 40% of extracted cannabinoids in some strains. It has mild sedative effects on its own, and demonstrates significant antipsychotic activity. It shines in combination with THC, where it decreases undesirable effects such as anxiety and paranoia. Cannabis indica is known for higher levels of CBD relative to THC, and has a reputation for a more relaxed “couch lock” high rather than the racing intensity of the sativas. A survey of cannabis users also found those exposed to both THC and CBD has significantly lower introvertive anhedonia and psychosis risk scores relative to those exposed only to THC.
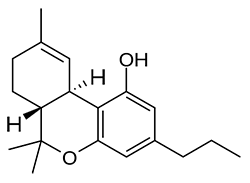
Tetrahydrocannabivarin (THCV) can be seen to be a close homologue of THC with a shorter sidechain. This psychoactive cannabinoid may be responsible for the differences in the unique high of “kush” and “haze” strains, and is found at levels of 5% to 50% of total cannabinoids in these strains. Interestingly enough THCV acts as an antagonist at the CB1 receptor, an action contrary to that of THC. This illustrates that the rich experience produced by cannabis cannot simply be isolated to a single compound.
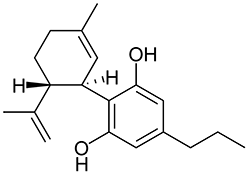
Cannabidivarine (CBDV) is related to CBD in the same way that THCV is related to THC, a homologue with a shortened sidechain. It does not display clear activity in man, but is the biosynthetic precursor to THCV in cannabis. It is possible to isomerize CBDV to THCV under acidic conditions, similar to the manner that CBD may be isomerized to THC. This “isomerization” procedure was popular in the 1970s, with machines sold in magazines that supposedly could accomplish this at home.
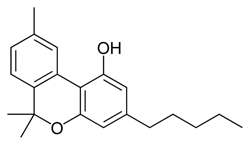
Cannabinol (CBN) is the village idiot of cannabinoids. There is relatively little of it in a fresh plant, as it is produced by degradation of THC. Over time and exposed to factors such as light and air, potent THC in stored cannabis will be slowly converted to relatively inactive CBN. This can be prevented by properly curing cannabis (ensuring controlled moisture levels) and storing it away from direct light in a sealed container such as a lightly packed mason jar.


Figured this was a great place to put this:
http://www.rexresearch.com/hhusb/hh6thc.htm
I was wondering if you could answer a question for me, Bill.
In this article, I quote “The boiling water treatment isomerizes the inactive CBD, and decarboxylates THCA to THC.”
Does direct heat (flame or conduction) have the same effect on the acid or is this a temperature sensitive process?
I don’t know – there’s a lot of talk about these methods, and most seem to stem from a pamphlet called “Dr. Atomic’s Marijuana Multiplier” and other related 70s texts. Now I don’t wish to insult the good doctor in any way, but I’m not sure these methods are as effective as claimed or else we would see more of this product for wide sale. Let me try to dig though some things and I’ll get back to you with a more definitive answer.
Edit: I’ve picked up a copy of “Marijuana Chemistry” by Micheal Stark which outlines some isomerization procedures - so it seems to not be fantasy. Perhaps the lack of isomerized product on the market a result of it being impractical for wide application, or the potency increase is marginal for the amount of work involved relative to modern methods like butane hash oil or bubble hash. I suspect that these methods are practical only for low quality cannabis which is not as prevalent today. Time to play around a bit. 🙂
Decarboxylation is a bit easier - 5 minutes in an oven at 200 degrees F should do it. I’ll put together a post or two on these subjects soon.
Edit 2: Here’s the decarboxylation post, Decarboxylation of Cannabis. Original title, I know.
Finally found a copy of “Dr Atomic’s Marijuana Multiplier“.
The increase in potency does arrive from conversion of CBD to THC. For lower potency strains of the 1970’s this process could be quite beneficial. For the boutique strains of today (ie 25%+ THC) there is simply not enough CBD present to produce potency gains of comparable magnitude.