Synthetic Cannabinoids: JWH-018 4-Alkyl Substitutions
Ah, JWH-018. Trivial to synthesize, highly potent, and a formerly legal substitute for the world’s most popular contraband drug. No wonder it reached the heights of popularity it did, and no wonder that labs are currently scrambling to replace it. And just like in corporate pharmacology, small substitutions can create not only a new compound, but a new cash cow free of previous legal restraints.
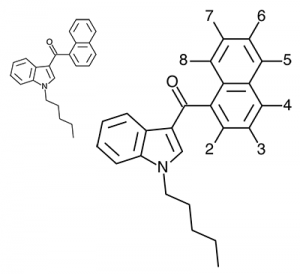
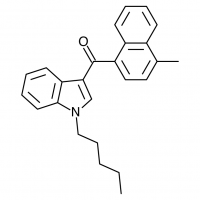
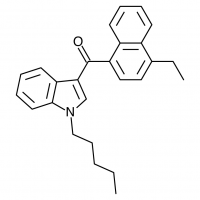
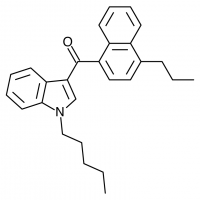
So let’s look at the naphthyl ring of JWH-018 in particular, and number our possible substitution sites for clarity. We could try many approaches at many positions, but suppose we limit ourselves to the 4-position. The halogens are a possibility (JWH-398 can be thought of JWH-018 with a chlorine substituted here), but let’s try some alkyl chains and see if they come up winners.
For reference, JWH-018 has binding affinities of 9.00 nM at CB1 and 2.94 nM at CB2. If we were a lab, we’d be looking for higher potency compounds (lower binding affinities) since increased attention from law enforcement means the only rational choice is to pack as much punch as you can in the smallest package for transport. It might be nice to try to search for compounds with the most pleasant effects, but that seems to be rather idealistic in the cold light of this new day.


No comments yet.