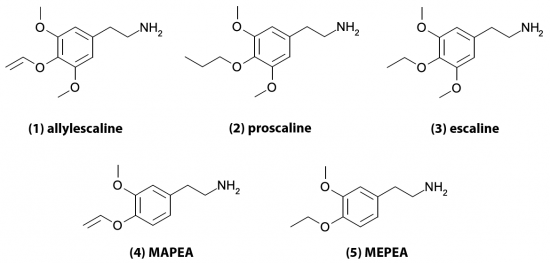
4-Hydroxy Tryptamines
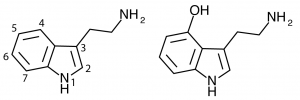
The indole ring of tryptamine provides a number of possible locations for functional groups to be substituted. Addition of a hydroxy group at the 4-position produces a large number of active psychedelic compounds including some true classics.
4-hydroxylation of alpha substituted tryptamines such as AMT has been conducted but further exploration has been limited due to potential toxic effects.
The 4-hydroxy analogue of α-MT has been looked at in human subjects. It is reported to be markedly visual in its effects, with some subjects reporting dizziness and a depressed feeling. There were, however, several toxic signs at doses of 15 to 20 milligrams orally, including abdominal pain, tachycardia, increased blood pressure and, with several people, headache and diarrhea.
-Alexander Shulgin
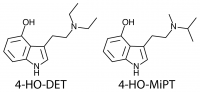
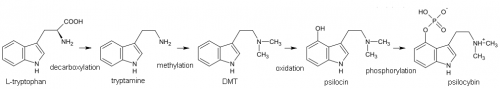
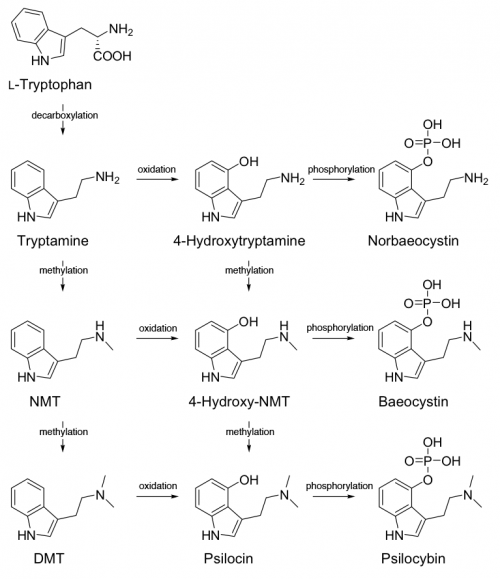
4-hydroxylation of the n-alkylated tryptamines is more fruitful. For instance, 4-hydroxylation of DMT (dimethyltryptamine) produces the classic psilocin (4-HO-DMT, 4-hydroxy-dimethyltryptamine). These 4-hydroxy n-alkyl tryptamines are similar in general psychedelic character, moderately potent (active at 10-25 mg) and of medium duration (2-6 hours).
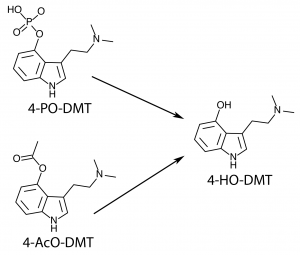
Other functional groups can be substituted at the 4-position which are converted to 4-HO tryptamines in the human body. Psilocybin (4-PO-DMT, 4-phosphoryloxy-dimethyltryptamine) contained in psychedelic mushrooms is water soluble and too polar to cross the blood-brain barrier. After consumption phosphatase enzymes rapidly break apart the phosphoryloxy group producing the active psilocin (4-HO-DMT, 4-hydroxy-dimethyltryptamine).
The phosphoryl group in psilocybin that is cleaved off by enzymes is known as an ester, and other esters can be substituted that react in similar ways once consumed by man.
O-acetylpsilocin (4-AcO-DMT, 4-acetoxy-dimethyltryptamine) can be thought of as psilocybin with an acetoxy group instead of a phosphoryloxy group. Like psilocybin, it is rapidly converted to 4-HO-DMT in the body. This produces a compound with a similar subjective experience to that of psilocybin.
4-HO tryptamines can therefore have a 4-AcO pair with very similar effects. The 4-AcO partner tends to be slightly less potent, have a longer duration, and be subjectively “smoother” than the 4-HO counterpart. It is a matter of debate whether this is simply the result of varying rates of administration due to metabolic conversion, or if 4-AcO tryptamines are active in their own right.
Shulgin, A. #48 AMT. Tryptamines I Have Known and Loved. Transform Press, 1997.
Vito Cozzi, Nicholas. MAPS: Re: Psilocybin and the blood brain barrier. MAPS Forum, April 29 2003.