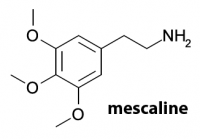
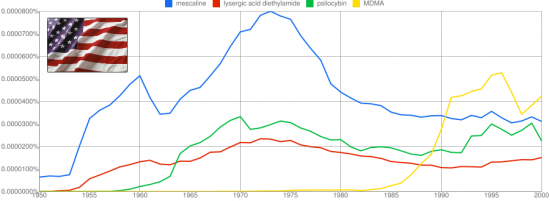
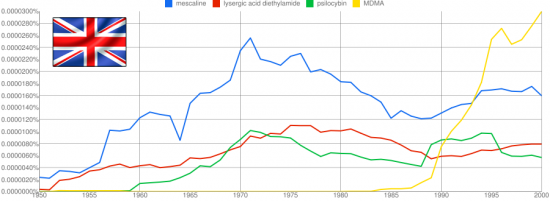
Leminger’s Scalines
Otakar Leminger was a little-known Czechoslovakian chemist who worked for years in industry and lived on the banks of the Elbe River in Ústí north of Prague. When he retired in the early 1970s he published a paper entitled “A Contribution to the Chemistry of Alkoxylated Phenethylamines” in which he describes the synthesis of several novel phenethylamines which he tested on himself to determine activity.
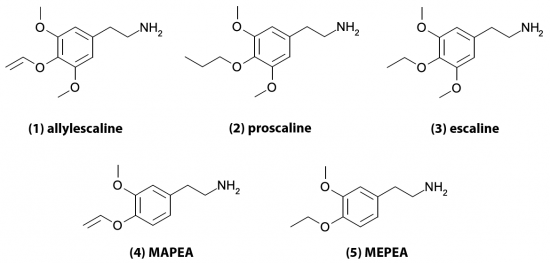
(1) allylescaline, 3,5-dimethoxy-4-allyloxy-phenethylamine (2) proscaline, 3,5-dimethoxy-4-n-propoxy-phenethylamine (3) escaline, 3,5-dimethoxy-4-ethoxy-phenethylamine (4) MAPEA, 3-methoxy-4-allyloxy-phenethylamine (5) MEPEA, 3-methoxy-4-ethoxy-phenethylamine
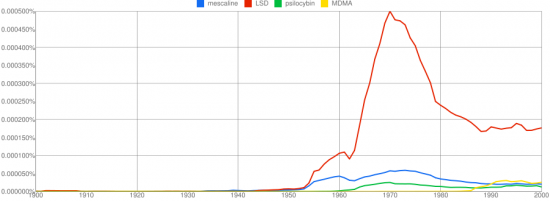
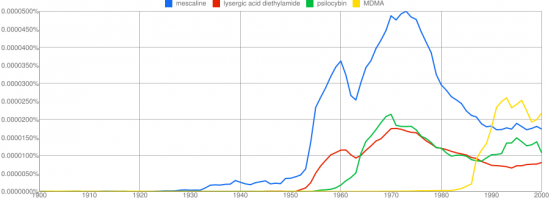
We can classify the compounds he discussed into two groups depending on the number of ring substitutions. Allylescaline, proscaline, and escaline have three while MAPEA and MEPEA have two. Generally phenethylamines with two ring substitutions are not active, but Leminger had found some exceptions. This knowledge might have been lost to time if not for the fact that Stanislov Wistupkin brought the paper to the attention of Alexander Shulgin.
[MAPEA and MEPEA are some] of the few phenethylamines with only two substituents that show even a hint of central activity. And there is an interesting story attached. I got a call out of absolutely nowhere, from a Stanislov Wistupkin, that he had discovered a number of new psychedelic drugs which he would like to share with me. They were simple phenethylamines, one with an ethoxy group at the 4-position, and one with an allyloxy group there. Both, he said, were mood elevators active between 100 and 300 milligrams. One of them was a material called MEPEA, and the other one was 3-methoxy-4-allyloxyphenethylamine, or MAPEA. When I did meet him in person, he gave me a most remarkable publication which had been authored some ten years earlier, by a person named Leminger, now dead. It was all in Czech, but quite unmistakably, right there on the third page, were the structures of MEPEA and MAPEA, and the statement that they were active at between 100 and 300 milligrams.
- Alexander Shulgin
MAPEA and MEPEA are only mildly active and interesting mostly in the sense that they appear to be the exception to the rule that phenethylamines with two ring substitutions are inactive. Leminger also created several mescaline variants with three ring substitutions by modifying the methoxy group at the 4 position and replacing it with an allyloxy, propoxy, or ethoxy group. The resulting compounds allylescaline, proscaline, and escaline were then tested on himself and found to be much more potent and intriguing.
Physiological effects of the compounds were examined only approximately on my body. The sulphate salts of MEPEA and MAPEA in doses 0.1-0.3 g were mild mood-elevators and were also cough calming agents. Allylescaline, proscaline, and escaline were much more active. Qualitatively there wasn’t a big difference among them and quantitatively their effect decreased: allylescaline was more potent than proscaline, and proscaline more potent than escaline. As an example the allylescaline experience is described:
“One hour after a 20 mg dose of allylescaline: perhaps slight vertigo, light drunkeness and pleasant excitation with locomotion need was observed. Eye perceptions were pricked up, colours seemed to be more warm and objects more plastic. Surroundings were much more interesting than usual. Colourful hallucinations were observed in the dark. Moreover, a calming effect to the breathing system and some kind of constriction of the digestive system was observed. Sleep at night was restless with megalomaniacal fantasies. Even 12 h after administration the effects were present. More serious studies of physiological activity are in contemplation.”
- Otakar Leminger
Leminger was the first to synthesize and consume allylescaline, the most potent of the mescaline derivatives explored. He was able to identify active phenethyamines with only two ring substitutions, a notoriously unproductive class of compounds. Did he conduct additional experimentation and screening beyond that detailed in this paper? No other publications by Leminger relating to psychedelic compounds are known.
Might there be other treasures that he had discovered, and never published? Was young Wistupkin a student of his? Are there unrecognized notes of Otakar Leminger sitting in some farm house attic in Northern Czechoslovakia? I extend my heartfelt salute to an almost unknown explorer in the psychedelic drug area.
- Alexander Shulgin
Otakar Leminger, A Contribution to the Chemistry of Alkoxylated Phenethylamines - Part 2. Chemicky Prumysl 22, 553 (1972).
Alexander Shulgin, #2 Allylescaline. Phenethylamines I Have Known and Loved, Transform Press (1991).
Alexander Shulgin, #123 MEPEA. Phenethylamines I Have Known and Loved, Transform Press (1991).