Grid Biosynthesis of Psilocybin
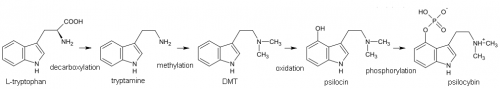
The biosynthesis of psilocybin in psychedelic mushrooms is a multi-step process, and the precise mechanism is debated by many authors. The essential amino acid l-tryptophan undergoes several modifying reactions (decarboxylation, N-methylation, 4-hydroxylation, and O-phosphorylation) but the specific order is unclear. A series of steps similar to the following is generally accepted.
Experiments with radiolabled precursors have shown that this is likely the primary path to psilocybin, however, labelled 4-hydroxytryptamine was also shown to be incorporated into the produced psilocybin indicating the possibility of an additional biosynthetic pathway. Other alkaloids present in psilocybin mushrooms such as baeocystin or norbaeocystin are not explained by this single pathway as well.
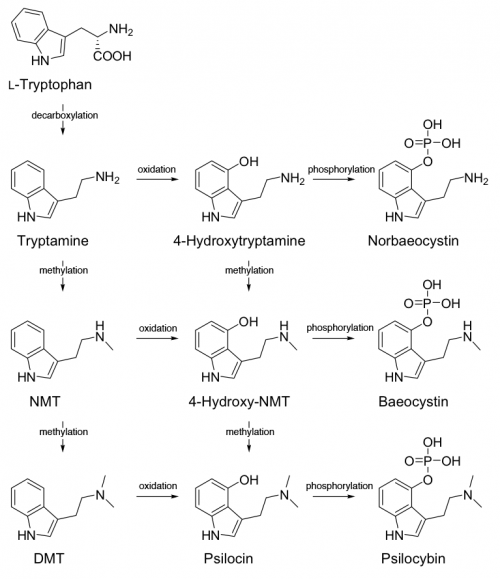
An elegant alternative has been proposed. What if instead of a single path and a set order of modifying reactions, there were multiple paths to psilocybin - with branching edges that led to baeocystin and norbaeocystin? The enzymes would compete and feed back among each other in a biosynthetic grid that preferred to produce psilocybin and psilocin but also produced small amounts of baeocystin and norbaeocystin as typically seen in nature.
There is no longer a preferred order to the modifying reactions, except for the obvious that 4-hydroxylation must precede O-phosphorylation. There are three paths to psilocin and psilocybin (the predominant alkaloids in psychedelic mushrooms), two paths to baeocystin (found in lesser concentrations than the two signature alkaloids), and one path to norbaeocystin (found in the lowest concentrations, if it is detectable at all). The number of paths does not indicate the absolute likelihood of producing a certain alkaloid, but it can be seen as a measure of resiliency. The precise weighting of each connection in the network is not clear at this point, or even if a steady state model would be an appropriate approximation.
Biosynthesis of Psilocybin. Part II: Introduction of Labelled Tryptamine Derivatives. S. Agurell and J. Lars G. Nilsson. Acta Chemica Scandinavica 22 (1968), 1210-1218.
Baeocystin and Norbaeocystin: New Analogs of Psilocybin from Psilocybe baeocystis. A.Y. Leung and A.G. Paul. Journal of Pharmaceutical Sciences, Vol. 57, No. 10, October 1968, 1667-1671.
Tryptamines as Ligands and Modulators of the Serotonin 5-HT2A Receptor and the
Isolation of Aeruginascin from the Hallucinogenic Mushroom Inocybe aeruginascens. Niels Jensen, Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultäten der Georg-August-Universität zu Göttingen, 2004.