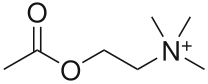
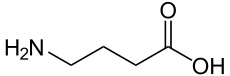
GABA
Nerve cells are designed to fire repetitively, and are subject to a barrage of stimuli. The brain prevents itself from spiraling out of control into hyperexcitable states by inhibition, a way of “turning down the volume” in the brain. The major workhorse for this is gamma animobutyric acid, or GABA. In a strange parallel, the brain synthesizes GABA in one step from glutamate, the brain’s major excitatory neurotransmitter. Excitation can therefore be efficiently followed by the capacity for inhibition, two naturally opposing processes depending on, and counterbalancing, the other. GABA and glutamate are the yin and yang of our nervous system.
There is hardly a single behavior that does not involve GABA. It is estimated to be involved in one third of all transmissions at synapses, and GABA nerve cells alone make up 40 percent of the total population of nerve cells, with glutamate cells adding perhaps another 30 percent. Broadly, the brain applies its fast acting GABA circuits at all levels to hold in check the local excitatory processes. Some GABA cells inhibit pivotal nerve cells that directly command other essential nerve cell populations. GABA mechanisms hold aggressive behavior in check in many animal species. If rats are made hyperactive by injection of dopamine into the nucleus accumbens, they will quickly quiet down after GABA is injected into the same nucleus.

GABA agonists include alcohol and benzodiazepines such as Xanax. Widely used for their tranquilizing, anti-anxiety, or sleep producing properties, they can create an incredibly risky state of addiction if used too frequently. Unlike other drugs that when withdrawn simply cause the perception of extreme suffering, cold turkey withdrawal causes GABA action to drop precipitously, inducing states of extreme agitation or convulsions, possibly causing death. A slow tapering of the drug, allowing natural GABA to rebound slowly, is the typically recommended course of action.
The inhibitory effects of GABA can be related to the “tunnel vision” experienced during states of drunkenness, where the visual field is reduced and attention is paid only to the objects directly in front of the subject. In contrast, bicluculline is a drug that stops the inhibitory role of GABA. When applied to the visual cortex, it expands the apparent visual fields by three to five times.
A traveller taking a benzodiazepine for a long haul flight and a frat boy on his 21st birthday both may experience periods of retrograde amnesia. This emphasizes the fact that when GABA systems become overactive, they can block the way a person accesses recently produced memories.
The anti-anxiety properties of GABA agonism are not limited to the “higher” civilized frustrations of the overworked executive as well. They can also relieve the deep primal anxiety of a baby monkey separated from his mother. Diazepam, a benzodiazepine, quickly stops their agitated behavior and cries of distress. High brain levels of dopamine are reduced, as well as high blood levels of ACh and cortisol which are correlated with stressful experiences.