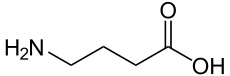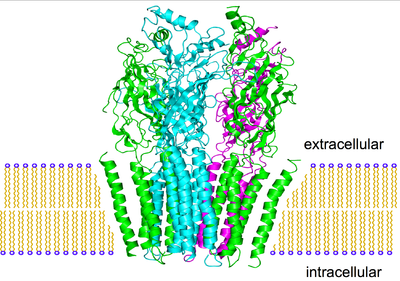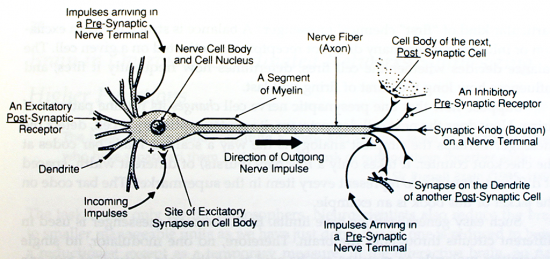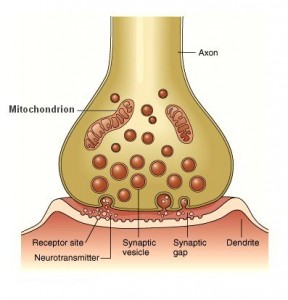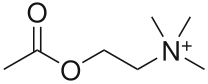
Acetylcholine (ACh) was the first neurotransmitter to be discovered. Identified in the year 1914 by Henry Hallett Dale for its actions on heart tissue, it was confirmed as a neurotransmitter by Otto Loewi. Both received the 1936 Nobel Prize in Medicine for their work. The nerve cells that release ACh are widespread and influential, involved in alertness, arousal, memory, and the fast brain waves which occur during waking and REM sleep.
In the peripheral nervous system, acetylcholine activates muscles, and is a major neurotransmitter in the autonomic nervous system. In the central nervous system, acetylcholine and the associated neurons form a neurotransmitter system, the cholinergic system, which is associated with anti-excitatory actions. ACh is also involved with synaptic plasticity, specifically in learning and short-term memory.
There are two main types of ACh receptors.
Nicotinic Acetylcholine Receptors

The first type of ACh receptor is called nicotinic, from the familiar compound nicotine of the tobacco plant. Nicotine was found to act as an ACh agonist, or compound that mimicked the action of ACh at these receptors. If you deliver nicotine to thalamic nerve cells, they fire instantly. Where are these nicotinic receptors prone to quick discharge? They reside in the medulla, hypothalamus, thalamus, in the cerebellum, and to a lesser degree in the cerebral cortex.
The stimulating effect of nicotine peaks within one minute after intravenous injection, wearing off a few minutes later. Any former smoker knows the pangs of nicotine withdrawal as the stimulating effect of nicotine is removed. Strangely enough, human subjects cannot distinguish between the euphoriant effect of nicotine and that of morphine or amphetamine. Does this imply that ACh agonism causes secondary release of other brain opioids or amines? The hypothesis is not well studied and is unresolved.
The primary effect is well established however. When nicotine activates presynaptic receptors, it causes a major enhancement of fast excitatory transmission. The immediate rush from smoking a cigarette appears to reflect the way that these nicotinic receptors are opening up the “nozzle” on other incoming terminals, allowing them to release much more of their fast acting ACh or glutamate transmitters into the synapse.
So what about antagonists, compounds that bind to the receptor but block action by not activating it? These include compounds used to cause peripheral muscle paralysis during surgery (as acetylcholine activates muscles, remember) and the antidepressant/smoking cessation aid bupropion. Bupropion binds to receptors and has a long half-life - so it sticks around, preventing any nicotine from binding to the receptors which would create a pleasurable response, making smoking cigarettes useless.
Muscarinic Acetylcholine Receptors

In the nineteenth century, the compound muscarine was isolated from the psychoactive mushroom Amanita muscaria. The mushroom itself in higher doses causes hallucinations, and was used intoxicant and entheogen by the peoples of Siberia. Muscarine was subsequently found to act as an ACh agonist on this class of receptors. They are located in diverse regions of the brain, and in contrast to the nicotinic receptors, begin to excite the next cell relatively slowly. For instance, a thalamic cell won’t increase its firing rate until 1.2 seconds after their incoming ACh receptors have been stimulated deep in the brain stem. But this slow start then develops momentum, as the thalamic cells continue to discharge for as long as 21 seconds. During this time, other thalamic cells become increasingly excited and develop fast, 35-45 Hz gamma rhythms. These rhythms may then be transmitted up to the cortex, as brain EEG scans begin to illustrate similar frequencies.
An opposing drug to muscarine is atropine, a muscarinic ACh antagonist. Named after Atropos, one of the three Fates in Greek mythology who chose how you were to die, atropine and related antagonists are found in plants such as belladonna and datura. Ingested in sufficient quantity, they cause strong and unpleasant deliriant effects with a distinct risk of death from toxicity or risky atypical behaviour. Even a modest blocking dose of atropine (1.25mg) demonstrates the risk of muscarinic antagonism. Subjects do not think as clearly, and struggle to maintain focused attention on even the simplest of tasks.