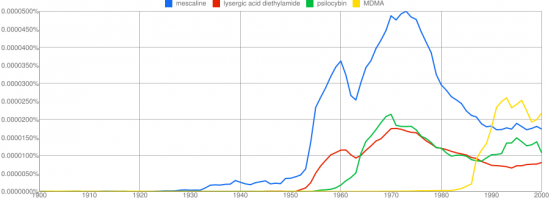
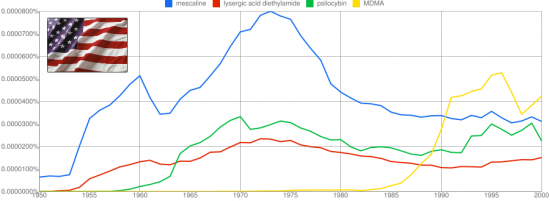
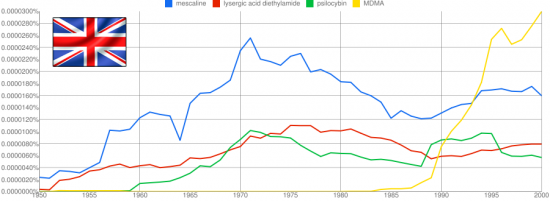
The 2C class of psychedelic compounds first researched by Alexander Shulgin encompasses a wide range of mental experiences, but one substitution in particular seemed to resonate with the magic seen in legendary compounds like mescaline. Thioalkylation at the 4-position produced the famed 2C-T-2 and 2C-T-7, compounds noted for an almost overwhelming visual character with echos of the cosmic choir, and a dismantling of the ego more prominent relative to the perceptually focused halogenated and alkylated 2Cs. This unique experience caused Shulgin to focus his substantial talents on sulfur based substitutions at the 4-position for a period of time, producing a large number compounds named in the format 2C-T-x. The first twelve are outlined in this post.
One attribute of these compounds must be emphasized at the outset. Unlike the halogenated or alkylated 2Cs, sulfur substitutions appear to be correlated with varying degrees of MAO inhibition, which can cause significant health issues up to and including death in extreme doses or in combination with other recreational drugs, particularly stimulants. These compounds were used safely in reasonable oral doses for twenty years among a qualified group of explorers, but the research chemical surge of the early 2000s introduced these compounds to an untrained audience in pure bulk forms. With resellers actively encouraged not to disseminate information on safer consumption due to legal pressures to maintain an appearance of “not for human consumption”, reckless dosing and routes of administration led to tragedy.
2C-T-2 has been sold widely, and has not been involved in any fatalities to my knowledge presumably due to less significant MAOI effect as judged by euphoric “push” relative to the other sulfur substitutions. 2C-T-7 can be considered the most popular of the group, and has nonetheless been involved in at least three deaths involving either excessive insufflated doses over 30mg, use in combination with stimulants such as MDMA or ephedrine, or both. The less popular 2C-T-21 has produced one death after an extreme oral dose (sticking tongue in full vial of pure compound).
The desire to experience the mental state produced by these compounds should be weighed with the intentions, ability, and risk tolerance of the end user. A society which allows the purchase of firearms with the assumption of responsibility should allow the same for self-exploration, but this is often not the case. If you are unfamiliar with the mechanism of risk of these compounds (ie MAO inhibition), if you are unable to weigh these compounds accurately, and if you are unwilling to respect the impact of route of administration, then an alternative should be selected.
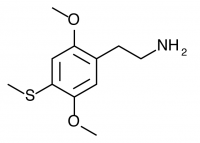
2C-T (2C-T-1, 2,5-dimethoxy-4-methylthiophenethylamine) Active in doses of 50-150mg, this compound produces a euphoric psychedelic experience for 5-6 hours that nonetheless was described as “generic” by Shulgin. Lack of potency relative to other closely related compounds and the somewhat shallow mental state make this a rare find on the research chemical market.
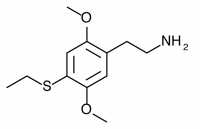
2C-T-2 (2,5-dimethoxy-4-ethylthiophenethylamine) Active in doses of 10-30mg, this compound produces a 6-8 hour experience most notable for its intellectual introspection, mescaline styled visuals, and unholy ability at higher doses to cause physical distress including nausea during the first hour of the experience. Like mescaline, after the purge a sense of contentment develops and the experience then progresses naturally. 2C-T-2 first rose to wide fame after the banning of 2C-B, and was first sold by Dutch smartshops as a substitute in 1997. As it produced a much richer and deeper psychedelic experience than 2C-B, it did not have as wide an appeal on the club circuit, but still sold widely enough that it was eventually banned by Dutch authorities.
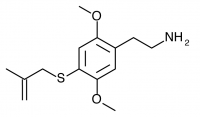
2C-T-3 (2C-T-20, 2,5-dimethoxy-4-(beta-methallyl)thiophenethylamine) Also known as 2C-T-20. Before working on the 2C-T series Shulgin investigated a similar series of promising compounds dubbed the Alephs, of which Aleph-3 was the beta-methallyl homologue. The synthesis of Aleph-3 was attempted, abandoned, and eventually forgotten. Years later the idea came to Shulgin again, and the beta-methallyl Aleph was begun anew along with the corresponding beta-methallyl 2C-T compound (2C-T-20). This led to the rediscovery of notes referencing the initial Aleph-3 synthesis attempt, and 2C-T-20 was renamed 2C-T-3 in order to maintain consistency with the Aleph project.
It is unclear if there was ever an “original” 2C-T-3 whose place was overwritten or shifted by insertion of the beta-methallyl. It is certainly an odd man out in the progression, as it would seem reasonable to place the n-propyl 2C-T-7 here after the ethyl 2C-T-2 and before the isopropyl 2C-T-4. A numbering system based on the previous Alephs appears to explain the gap, as perhaps the initial synthesis of the 2C-T-3 was simply left for later due to the increased difficulty relative to the alkyls surrounding it. The synthesis of 2C-T-3/2C-T-20 has never been completed according to public knowledge.
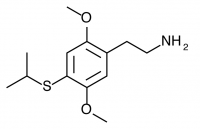
2C-T-4 (2,5-dimethoxy-4-isopropylthiophenethylamine) The isopropyl companion to the propyl substituted 2C-T-7. Active in doses between 8-20mg, it produces a long lasting change in mental state for 12 to 18 hours with a slight dissociative character. Human testing showed huge variance in responses, particularly in the subcategories of visual distortion and euphoric response. The inability to predict whether a 12+ hour long psychedelic experience would be euphoric and illuminating or dysphoric and anxiety ridden has contributed to a lack of recreational usage of this drug.
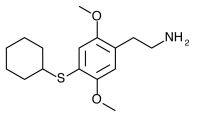
2C-T-5 (2,5-dimethoxy-4-cyclohexylthiophenethylamine) Has never been synthesized according to public knowledge, and was presumably based the similarly structured Aleph-5.
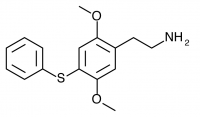
2C-T-6 (2,5-dimethoxy-4-phenylthiophenethylamine) Has never been synthesized according to public knowledge, and was presumably based the similarly structured and successfully synthesized Aleph-6.
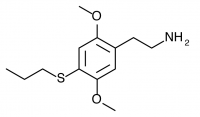
2C-T-7 (2,5-dimethoxy-4-(n)-propylthiophenethylamine) Active in doses of 10-40mg with a duration of 8-16 hours, 2C-T-7 is perhaps the most popular of Shulgin’s sulfur substitutions likely due to a more consistent euphoric effect. A powerful psychedelic character lies underneath however, and initial hype about a easy to handle “candyflip” experience may have resulted in some overwhelming experiences. With nausea reported less frequently as a side effect in comparison to 2C-T-2, many cast 2C-T-2 aside as a shorter less euphoric 2C-T-7. This is not necessarily a valid critique, as the more euphoric mental state of 2C-T-7 often results in “sloppier” emotional analysis relative to the slightly more difficult and intellectual 2C-T-2. 2C-T-7 began its rise to fame in the summer of 1999 as Dutch smartshops began selling it in 7.5mg tablets dubbed “Blue Mystic” due to the success (and resulting ban) of 2C-T-2. Unsurprisingly, 2C-T-7 was quickly banned as well.
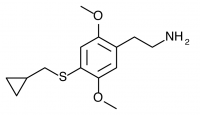
2C-T-8 (2,5-dimethoxy-4-cyclopropylmethylthiophenethylamine) Active in doses of 30-50mg and producing a 10-15 hour experience, this compound produces a mental state that many did not wish to repeat. Shulgin said in PiHKAL, “there are as many negatives as there are positives, and the particular substitution pattern is not one to set the world on fire.”
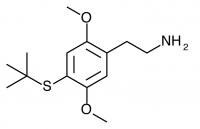
2C-T-9 (2,5-dimethoxy-4-(t)-butylthiophenethylamine) Active in doses of 60-100mg, it produces a 12-18 hour experience with significant peripheral body load and little psychedelic reward for the effort. Note that Wikipedia currently incorrectly refers to this as the n-butylthio substitution rather than the tert-butylthio referenced in PiHKAL, as the n-butylthio substitution should be referred to as 2C-T-19.
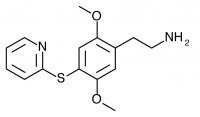
2C-T-10 (2,5-dimethoxy-4-(2-pyridylthio)phenethylamine) Synthesis was abandoned before completion, and has not been completed by others according to public knowledge.
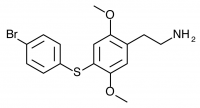
2C-T-11 (2,5-dimethoxy-4-(4-bromophenylthio)phenethylamine) Synthesis was abandoned before completion, and has not been completed by others according to public knowledge.
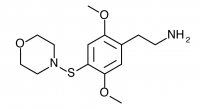
2C-T-12 (2,5-dimethoxy-4-(1-morpholinothio)phenethylamine) Synthesis was abandoned before completion, and has not been completed by others according to public knowledge.
Sulfurous Samadhi, An Investigation of 2C-T-2 & 2C-T-7 by Murple, Feb 6, 2001.
Nichols, D. E. and Shulgin, A. T. (1976), Sulfur analogs of psychotomimetic amines. Journal of Pharmaceutical Sciences, 65: 1554–1556. doi: 10.1002/jps.2600651040
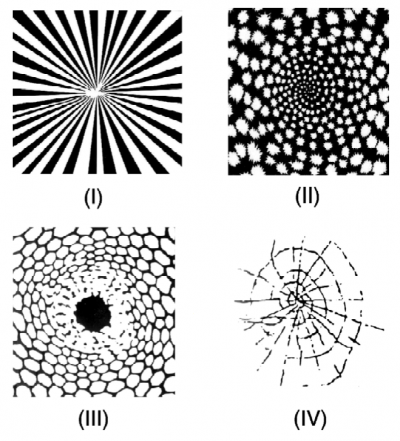
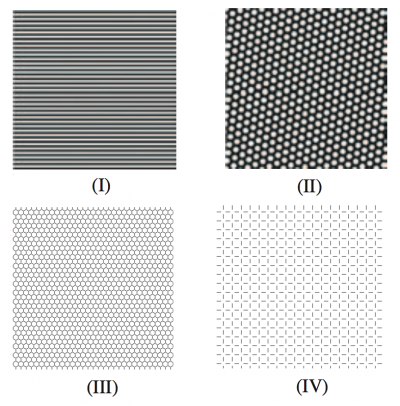
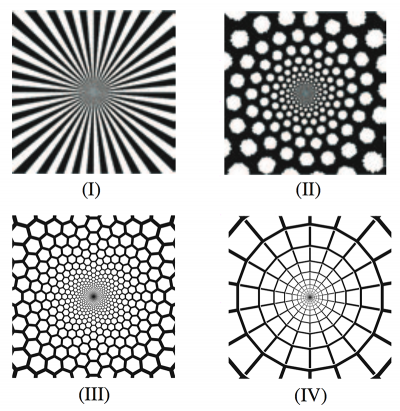
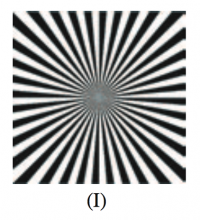 Form constants are archetypical visual patterns generated by noise in the visual cortex which is then twisted by the wiring between retina and brain. Type I form constants were described as “tunnels” by Kluver, and postulated to be generated by the non-contoured roll noise pattern described by Cowan and Ermentrout. I decided to visualize this model in higher resolution to further explore the generated forms. All of the following use sinusoidal noise to approximate visual cortex noise for the first half of the video, and then show the transformed result of the mind’s eye for the latter half of the video. The direction of travel of noise across the complex plane can be shown to produce three form constant subtypes.
Form constants are archetypical visual patterns generated by noise in the visual cortex which is then twisted by the wiring between retina and brain. Type I form constants were described as “tunnels” by Kluver, and postulated to be generated by the non-contoured roll noise pattern described by Cowan and Ermentrout. I decided to visualize this model in higher resolution to further explore the generated forms. All of the following use sinusoidal noise to approximate visual cortex noise for the first half of the video, and then show the transformed result of the mind’s eye for the latter half of the video. The direction of travel of noise across the complex plane can be shown to produce three form constant subtypes.