The Alkylated 2Cs
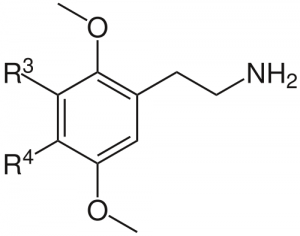
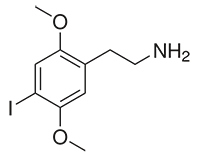
Alexander Shulgin investigated a large number of substituted phenethylamines, and dubbed one class with methoxy groups substituted on the 2 and 5 positions of the benzene ring the “2C”s, for the two carbon atoms between this ring and the amino group. A suprisingly large number of substitutions on the 3 and 4 positions demonstrated intruiging psychedelic activity, including halogenation at the 4 position. There are other possible substitutions as well, including the alkylated 2Cs, which are generally considered “deeper” psychedelics than their halogenated cousins, with complex visuals and a more analytical and challenging psychedelic state.
|
3 position: hydrogen 4 position: alkyl groups, consisting of only single bonded carbon and hydrogen atoms (methyl, ethyl, propyl, isopropyl) |
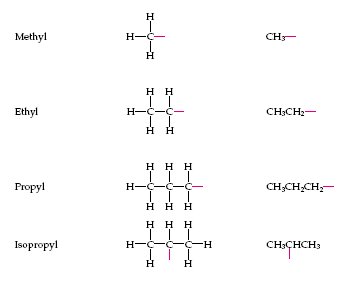 |
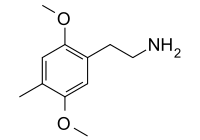
2C-D (2,5-dimethoxy-4-methylphenethylamine) has the simplest alkyl substitution, a methyl group. Personally, I’m a bit confused why this isn’t called 2C-M, but I don’t get to name these things. Best known as as a “smart drug” at doses under 10mg, it has been reported to aid in learning but has produced inconsistent results in trials. Shulgin referred to it as “pharmacological tofu”, a substance that could be used to potentiate and extend the action of other materials without changing the experience significantly - similar to the manner in which tofu in a stirfry absorbs the surrounding flavors without contributing a significant taste of its own. At higher doses of 30-100mg it is a proper psychedelic in its own right, but this lower potency has contributed to a lack of relative popularity due to a higher cost per dose.
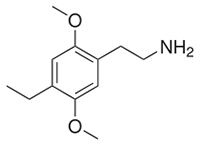
2C-E (2,5-dimethoxy-4-ethylphenethylamine) is currently the most well known of the alkylated 2Cs, a material Shulgin dubbed “difficult but worthwhile”. Typically taken orally in doses of 10-25mg, it produces a psychedelic state lasting 8-12 hours described as emotionally neutral by many. The dose-response curve is also significantly steeper than 2C-D, with 20mg subjectively producing an experience twice as powerful as 15mg, and quickly spiraling to undesirably intense states above 25mg. As one of the most visual and powerfully introspective phenethylamines, it has gained favor with those preferring the deeper psychedelic states produced by compounds such as LSD.
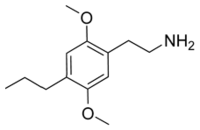
2C-P (2,5-dimethoxy-4-propylphenethylamine). As the length of the alkyl chain increases, potency and duration increase along with it. Oral doses of 5-15mg begin to show effect after 3 hours, and can last 10-16 hours. While the visual style is similar to 2C-E, the mental state is slightly deeper with a more stimulated feel. Doses of 2C-P were regarded with great caution due to a concise note by Shulgin indicating that 16mg was “clearly an overdose”. This cast fear in some and doubt in others, as no apparent toxic effects had been noted with dosage levels approaching this, although the dose-response curve for mental effects appeared even steeper than 2C-E. Shulgin responded to the statement in 2004 with the following clarification:
I contacted the person who tried the 16 milligram dose of 2C-P and who gave me the phrase, “16 mg was clearly an overdose, with the entire experiment labeled a physical disaster, not to be repeated.”
He checked his notes and shared more details that establishes context for this comment. When he was about eleven years old, he was sitting in his living room of his parent’s house, and a medium sized earthquake happened and knocked over some furniture including a full bookcase. This landed on his legs and hurt him very badly. Nothing was broken, but the pulled muscles made it painful to walk. This discomfort lasted for upwards of a year.
At the peak of the 2C-P trial (he about 45 years old at the time) he recalled and re-lived this frightening experience and there was extreme pain in his legs. No cardiovascular effects, or signs of toxicity. It was a childhood physical disaster that was re-lived and he had no wish to repeat that particular dose, as he didn’t want to repeat the pain. He did another experiment two weeks later, with 9 milligrams, and had a long-lived +++ but had no childhood memories!
This all took place in late 1985.
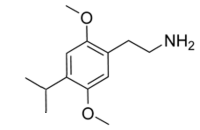
2C-iP (2,5-dimethoxy-4-isopropylphenethylamine) has had little testing in humans, was not investigated in PiHKAL, and existing reports are inconsistent. Some suggest it is effectively inactive, with a large (1-2 orders of magnitude) decrease in potency relative to 2C-P (similar to the relationship of DOIP to DOPR), while others describe an experience similar to 2C-E requiring 1.5x the material for a comparable (although longer lasting) experience. It seems likely that more reports will appear soon, and perhaps the effects of this rare material will become more clear with time.
What about the longer alkyl chains (butyl, pentyl, etc.)? To my knowledge, they have not been sufficiently tested in humans to draw any real conclusions about their activity at this point in time. Shulgin found that activity declined significantly with the butyl group in the substituted amphetamines (DOM ~ DOET ~ DOPR > DOBU), but the action in the 2C compounds may not be quite as clear. Combined with the increase in difficulty of synthesis and the infamous maxim “There’s ethyl and propyl, but butyl is futile”, 2C-BU and other related compounds are unlikely to gain popularity absent some surprises. In general, the alkylated 2Cs share a complex style of visual distortion and a similar psychedelic character - but potency per unit weight, duration, and steepness of the dose-response curve increase as the size of the first three normal alkyl groups grows.