Acid · Lysergide · d-Lysergic acid diethylamide · METH-LAD · d-Lysergamide, N,N-diethyl · N,N-Diethyl-d-lysergamide · 9,10-Didehydro-N,N-diethyl-6-methylergoline-8β-carboxamide
A suspension of 3.15 g d-lysergic acid hydrate and 7.1 g of diethylamine in 150 mL CHCl3 was brought to reflux with stirring. With the external heating removed, there was added 3.4 g POCl3 over the course of 2 min, at a rate sufficient to maintain refluxing conditions. The mixture was held at reflux for an additional 5 min, at which point everything had gone into solution. After returning to room temperature, the solution was added to 200 mL of 1 N NH4OH. The phases were separated, the organic phase dried over anhydrous MgSO4, filtered, and the solvent removed under vacuum. The residue was chromatographed over alumina with elution employing a 3:1 C6H6/CHCl3 mixture, and the collected fraction stripped of solvent under hard vacuum to a constant weight. This free-base solid can be recrystallized from benzene to give white crystals with a melting point of 87–92 °C. IR (in cm-1): 750, 776, 850, 937 and 996, with the carbonyl at 1631. The mass spectrum of the free base has a strong parent peak at mass 323, with sizable fragments at masses of 181, 196, 207 and 221.
This base was dissolved in warm, dry MeOH, using 4 mL per g of product. There was then added dry d-tartaric acid (0.232 g per g of LSD base), and the clear warm solution treated with Et2O dropwise until the cloudiness did not dispel on continued stirring. This opaqueness set to a fine crystalline suspension (this is achieved more quickly with seeding) and the solution allowed to crystallize overnight in the refrigerator. Ambient light should be severely restricted during these procedures. The product was removed by filtration, washed sparingly with cold methanol, with a cold 1:1 MeOH/Et2O mixture, and then dried to constant weight. The white crystalline product was lysergic acid diethylamide tartrate with two molecules of methanol of crystallization, with a mp of about 200 °C with decomposition, and weighed 3.11 g (66%). Repeated recrystallizations from methanol produced a product that became progressively less soluble, and eventually virtually insoluble, as the purity increased. A totally pure salt, when dry and when shaken in the dark, will emit small flashes of white light.
DOSAGE: 60 to 200 micrograms, orally
DURATION: 8–12 hrs h
QUALITATIVE COMMENTS:In the case of LSD, it seems presumptuous to attempt to select typical comments for quotation. Literally thousands of reports are in the literature, from early exploratory research, to clinical applications for treatment of autism, of alcoholism, or mental illness, to assisting in psychotherapy and in the dying process, to the adventures of the military in both intelligence and chemical warfare, to innumerable anecdotal tales of pleasure and pain. Dozens of books have been devoted to these topics.
EXTENSIONS AND COMMENTARY: LSD is an unusually fragile molecule and some comments are in order as to its stability and storage. As a salt, in water, cold, and free from air and light exposure, it is stable indefinitely. There are two sensitive aspects of its structure. The position of the carboxamide attachment, the 8-position, is affected by basic, or high pH, conditions. Through a process called epimerization, this position can scramble, producing isolysergic acid diethylamide, or . This product is biologically inactive, and represents a loss of a proportionate amount of active product. A second and separate point of instability is the double bond that lies between this 8-position and the aromatic ring. Water or alcohol can add to this site, especially in the presence of light (sunlight with its ultraviolet energy is notoriously bad) to form a product that has been called , which is totally inactive in man. Oh yes, and often overlooked, there may be only an infinitesimal amount of chlorine in treated tap water, but then there is only an infinitesimal amount of LSD in a typical LSD solution. And since chlorine will destroy LSD on contact, the dissolving of LSD in tap water is not appropriate.
There are many synthetic methods developed and reported for the preparation of LSD. All of them start with , and for that reason it has been listed as a Schedule III controlled drug, as a depressant, under Federal law. The amide lysergamide, a component of several varieties of morning glory seed, is also a controlled drug and, by law, a depressant. The earliest syntheses of LSD involved the used use of an azide intermediate (the original Hofmann process, 1955), mixed anhydrides with trifluoroacetic anhydride (1956) or sulfuric anhydride (SO3-DMF on the lithium salt, 1959), with the peptide condensation agent N,N′-carbonyldiimidazole (1960), or with the acid chloride as the active intermediate with POCl3, PCl5 or thionyl chloride (1963) or just phosphorus oxychloride (1973). Most methods are faulted due to excessive moisture sensitivity, generation of side-products, or epimerization or inversion at the 8-position carbon to form . The POCl3 procedure is clean and fast, and is the preferred process today for the synthesis of a wide variety of substituted lysergamides.
The term LSD comes from the initials of the German for lysergic acid diethylamide, or Lysersäure Diethylamid LyserSäure Diäthylamid. The number “25” following it has many myths attached to it, such as it was the 25th form of LSD that Hofmann tried, or it was his 25th attempt to make LSD. From my own experience with chemical companies that are allied with pharmaceutical houses, I had assumed that the chemical name (which might be a mouthful for the pharmacologist) was simply replaced with a pronounceable code number equivalent. But the answer here is yet simpler. Hofmann, in his LSD, My Problem Child wrote: “In 1938, I produced the twenty fifth substance in a series of derivatives: lysergic acid diethylamide, abbreviated LSD-25… for laboratory usage.”
Within a few years of the discovery of the extraordinary potency of LSD, a large number of close analogues were synthesized by Hofmann and his allies at Sandoz. Over the following decade many were tested in humans, both in patients and healthy subjects, with the qualitative descriptions and dosages published in the medical literature.
A number of analogues of LSD have maintained the diethylamide group unchanged, but additions or changes have been made in the pyrrole ring.
| —indole-ring substituent— | |||
|---|---|---|---|
| at N-1 | at C-2 | Code code | name |
| H | H | LSD-25 | N,N-Diethyllysergamide |
| COCH3 | H | ALD-52 | |
| CH3 | H | MLD-41 | |
| CH2OH | H | OML-632 | |
| CH2N(CH3)2 | H | ||
| H | -B -Br | BOL-148 | |
| H | I | ||
| CH3 | Br | MBL-61 | |
| CH3 | I | MIL | |
There are some suggestions that an intervenous intravenous route may be more effective. I have heard of effects being noted at maybe a milligram and a short (2–3 hour) intoxicaion intoxication following 20 milligrams administered over a 20 minute period. I was involved many years ago in a study of radio-labelled which was made by the bromination of LSD. I was quite sure that the only radioactive material present was BOL-148, but there could well have been some unreacted LSD still present which would, of course, still be psychoactive. The synthesis is not clean—I was tempted to make an entry for this compound if only to reproduce Albert Hofmann’s original published experimental procedure. He reacted 13.2 grams of N-bromosuccinimide (in 400 mL dioxane, with 1.2 liters of dioxane containing 25 grams of LSD. This gave 11 grams of crude produce product which had to be recrystallized. The radioactive syntheses uses effectively elemental bromine, and gave yields of from 5 to 15%. Visualize that reaction! A warm flask containing over a quart of warm solvent in which there was maybe half a million doses of LSD.
These three additional compounds are shown here because they were described in a synthetic flurry that followed the discovery the activity of LSD. But at the moment I know neither their internal Sandoz codes nor if they had ever been explored in man. This is a kind of frustrating catch-all entry, in that the long index will send you here, and once here you realize that nothing is known. Well, at least the compounds are known, and perhaps there is something in the Sandoz vaults that might be interesting. I do not have access to them.
I was at a meeting of a NIDA study section a few years ago, where some one presented some findings with a group of subjects who were complaining of continuing mental problems alledgedly allegedly due to LSD exposure. A chart was put up showing the outline of the brain showing the locations of the EEG foci that were observed in one of these subjects. Along side it was a PET scan showing the distribution of radioactive LSD in a subject. The purpose was to discuss the similarities and differences of the coordinates of electrical activity and radio-isotope concentration. I innocently asked what positron isotope had been used, as I did not know of any successful positron labelling of LSD. Carbon 11, I was told. Where in the molecule was the label incorporated, I asked. In the 1-position methyl group.  It was finally acknowledged that the compound that had actually been used was 2-Iodo-1-methyl-LSD, our compound, which is quite a different world. A pharmacologist might say that they are similar in action (looking at , not psychedelic action), and achemist a chemist might say they are of similar structure (looking at the upper 80% of the moledule molecule. But they are different compounds. This is a most subtle form of deceit. It is, in fact, out and out dishonest, but it looks good up there on the screen at a lecture.
It was finally acknowledged that the compound that had actually been used was 2-Iodo-1-methyl-LSD, our compound, which is quite a different world. A pharmacologist might say that they are similar in action (looking at , not psychedelic action), and achemist a chemist might say they are of similar structure (looking at the upper 80% of the moledule molecule. But they are different compounds. This is a most subtle form of deceit. It is, in fact, out and out dishonest, but it looks good up there on the screen at a lecture.
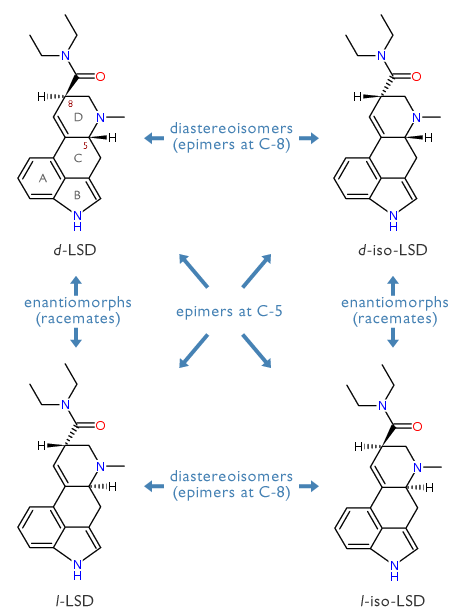
Let me mention in passing, that there are three stereoisomers possible for d-LSD. There are , , and . The inversion of the stereochemistry of the attached diethylcarboxyamido group of d-LSD gives the diastereoisomer (d-iso-LSD) which is a frequent synthetic impurity of d-LSD itself. The corresponding optical antipodes l-LSD and l-iso-LSD are also known and have been tasted. All three are completely inactive: d-iso-LSD shows no psychological changes at an oral dose of 4 milligrams; l-LSD none at up to 10 milligrams orally; and l-iso-LSD none at 500 micrograms orally. These dramatic decreases in potency show both the stereoselectivity of the native LSD molecule in producing its central effects, and the LSD-free purity of these isomers.
LSD Stereoisomers
The second major location of variations in the structure of LSD has been in the nature of the alkyl groups on the amide nitrogen atom. Some of these are Sandoz syntheses, some are from other research groups, and a few of them are found in nature. Some of these have been studied in man, and some have not. A few of the original clutch of Sandoz compounds have both 1-substituents and amide alkyl (R) group variations:
| indole | — amide nitrogen substituents — | |||
|---|---|---|---|---|
| R= | R= | R= | code name |
chemical name |
| H | H | H | LA-111 | |
| H | CH3 | H | ||
| H | CH2CH3 | H | LAE-32 | |
| H | (CH2)2CH3 | H | ||
| H | CH(CH3)CH2OH | H* | Ergonovine | |
| H | CH(CH3)CH2OH (CH2)3CH3 | H | ||
| H | (CH2)4CH3 | H* | ||
| H | CH(CH3)CH2CH2OH | H* | Methergine | |
| H | (CH2)4CH3 | H | ||
| H | CH(CH3)CH2CH2CH3 | H* | ||
| H | CH(CH2CH3)2 | H | ||
| H | (CH2)5CH3 | H | ||
| H | CH(CH3)CH2CH2CH2CH3 | H* | ||
| H | (CH2)6CH3 | H | ||
| H | CH(CH3)CH2CH2CH2CH2CH3 | H* | ||
| H | CH3 | CH3 | DAM-57 | |
| H | CH2CH3 | CH3 | ||
| H | (CH2)2CH3 | CH3 | LAMP LMP |
|
| H | CH(CH3)2 | CH3 | ||
| H | CH(CH3)CH2C6H5 | CH3* | ||
| H | CH2CH3 | CH2CH3 | LSD-25 | N,N-Diethyllysergamide |
| H | (CH2)2CH3 | CH2CH3 | ||
| H | (CH2)2CH3 | (CH2)2CH3 | ||
| H | CH(CH3)2 | CH(CH3)2 | ||
| H | CH2CH=CH2 | CH2CH=CH2 | DAL | |
| H | (CH2)3CH3 | (CH2)3CH3 | ||
| H | -CH2CH2CH2CH2- | LPD-824 | ||
| H | -CH2CH=CHCH2- | |||
| H | -CH2CH2CH2CH2CH2- | |||
| H | -CH2CH2OCH2CH2- | LSM-775 | ||
| CH3 | CH2CH3 | H | MLA-74 | |
| CH3 | CH(CH2CH3)CH2OH | H* | UML-491 | |
| COCH3 | CH2CH3 | H | APA-10 ALA-10 | |
| CH3 | -CH2CH2CH2CH2- | MPD75 MPD-75 |
||
In the amides marked with “*” there has been the introduction of a new asymmetric center, which of course doubles the number of isomers that is possible. In each case the resulting two optical forms were prepared separately, and evaluated separately as to their pharmacology.
This listing is not intended to be thorough, but it is shown to suggest the amount of synthetic effort that has been made towards the exploring and understanding the high potency associated with those two remarkably important ethyl groups on the amide nitrogen of LSD. I have given the Sandoz code names, again, as far as I know them. Although none of these really warrant a dedicated recipe, there is sufficient animal and human pharmacology reported to justify listing them below as separate items. Most of these reports appeared in the mid-1950’s but some studies are still being done and papers are published even today with new ideas but, sadly, only with animal pharmacology. I have been as guilty as the next person who has tried to mount all these compounds into a table with a “human potency” factor that compares them directly to LSD. This is an uncomfortable simplification. Here are the actual reported observations, and I’ll let the reader provide his own potency index.
The third location of structural modification of the LSD molecule has been at the 6-position in ring D. This is the LAD series with any of several alkyl groups attached to the nitrogen atom. The methyl group is found with LSD itself, and is reason for using METH-LAD in the title as a synonym. The ethyl, allyl and propyl substitutions provide , , and , and each of these commands a separate entry.
The most frequently encountered precursor for the manufacture of LSD is ergotamine, a major alkaloid of the ergot world. It is totally unknown in the morning glories. The usual commercial form is the tartrate salt, and is often referred to under the code abbreviation of ET, for ergotamine tartrate. It has found medical use in the treatment of migraine headaches, and as an oxytocic (an agent that is used in childbirth to stimulate uterine contractions. Care with the ET terminology must be taken, in that in the drug world it has two additional associations; for alpha-ethyltryptamine and for N-monoethyltryptamine.
is a naturally occurring, water-soluble ergot alkaloid, found in both ergot preparations and in many species of morning glory seeds, and there are several reports of LSD-like action at oral levels of between two and ten milligrams. It has an important use in obstetrics, again as an oxytocic, at about a tenth of this dose. This pharmacological potential must be respected in psychopharmacological trials. The one-carbon homologue (the butanolamide rather than the propanolamide) is called or methylergonovine. It is a synthetic ally and is orally effective as an oxytocic at a dosage of 200 micrograms. It also has an LSD-like action at ten times this level.
Although there are many other chemical treasures in the ergot fungal world, I would like to wrap this commentary up with a return to the topic of morning glory seeds. Four additional alkaloids of the ergot world must be acknowledged as being potentially participating factors in the MGS story. With each of these, the primary ergoline ring system is largely intact but the amide function is completely gone. The carboxyl group has been reduced to the alcohol to give . There is the related molecule present which is the isomer with the double bond moved to be conjugated with the aromatic ring; it is called . There is the same molecule but with a hydroxy group attached to the 8-position carbon atom (an ethyleneglycol!); it is called . And lastly, that D-ring can actually be opened between the 5 and 6 positions, to give us a secondary amine tryptamine derivative, . To be completely anally retentive in this Ipomoea inventory, mention must be made of five alkaloids that are present in truly trace amounts, all of which have no oxygen atoms present whatsoever on that substitution on the ergoline 8-position. These are the 8-methyl isomers , , and , and the methylene analogue . These structures in effect define absolute obscurity, and most probably do not contribute to the morning glory intoxication state. But the others, some present is sizable amounts, may someday help explain why the pharmacology of these seeds is so different than that of the major isolates, the ergines.
20 Jun 2018 · · Isomer Design
About TiHKAL · info
This version of Book II of TiHKAL is based on the Erowid online version created by Bo Lawler with the help of Erowid, from content generously provided in electronic format by the Authors.
The Erowid online version does not always align precisely with the printed version. Text appears to have been inserted, deleted, or changed at various points. Where the two are seen to diverge both the Erowid and print versions are given. Sharp-eyed readers are encouraged to report novel discrepancies.
As with PiHKAL, I’ve again attempted to reproduce the typographic style of the printed edition. I’ve again made minor changes to some chemical names in line with current nomenclature practice. Typically the change is little more than expanding a prefix or setting it in italics. The history page has further details.
Cautionary note
“At the present time, restrictive laws are in force in the United States and it is very difficult for researchers to abide by the regulations which govern efforts to obtain legal approval to do work with these compounds in human beings.“No one who is lacking legal authorization should attempt the synthesis of any of the compounds described in these files, with the intent to give them to man. To do so is to risk legal action which might lead to the tragic ruination of a life. It should also be noted that any person anywhere who experiments on himself, or on another human being, with any of the drugs described herein, without being familiar with that drug’s action and aware of the physical and/or mental disturbance or harm it might cause, is acting irresponsibly and immorally, whether or not he is doing so within the bounds of the law.”
Copyright notice
The copyright for Book I of TiHKAL has been reserved in all forms and it may not be distributed. Book II of TiHKAL may be distributed for non-commercial reproduction provided that the introductory information, copyright notice, cautionary notice and ordering information remain attached.
Ordering information
TiHKAL is the extraordinary record of the authors’ years exploring the chemistry and transformational power of tryptamines. This book belongs in the library of anyone seeking a rational, enlightened and candid perspective on psychedelic drugs.
Though Sasha and Ann have put Book II of TiHKAL in the public domain, available to anyone, I strongly encourage you to buy a copy. We owe them — and there’s still nothing quite like holding a real book in your hands.
TiHKAL (ISBN 0-9630096-9-9) is available for US$24.50 (plus $10 domestic first-class shipping) from Transform Press.
Transform Press,Box 13675
Berkeley, CA 94701
510 · 934 · 4930 (voice)
510 · 934 · 5999 (fax)