Biosynthesis of 4-Substituted Tryptamine Derivatives
 Biological organisms are wondrous little molecular factories, their enzyme catalyzed reactions often accomplishing in a single step what would confound a chemist in a well-stocked laboratory. Researchers have attempted to harness these biosynthetic pathways to create complex molecules not easily synthesized by conventional methods.
Biological organisms are wondrous little molecular factories, their enzyme catalyzed reactions often accomplishing in a single step what would confound a chemist in a well-stocked laboratory. Researchers have attempted to harness these biosynthetic pathways to create complex molecules not easily synthesized by conventional methods.
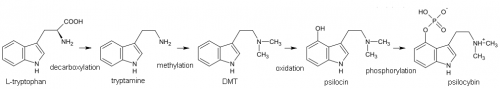
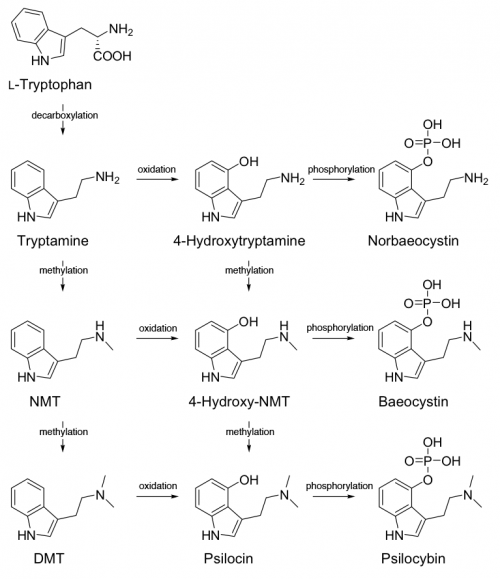
Psilocybin is produced via a biosynthetic grid where enzymes act on various closely related intermediate compounds in turn. The enzymes do not appear to be particularly picky about the compounds they modify. For instance, dimethyltryptamine (DMT) is hydroxylated to 4-HO-DMT naturally in psilocybin mushrooms. Other precursor compounds like tryptamine and methyltryptamine are also hydroxlyated to 4-hydroxy-tryptamine and 4-hydroxy-methyltryptamine respectively.
If an entirely new synthetic tryptamine of similar structure was introduced to these mushrooms, would the same enzymes act on it? This could produce a new and unique psychedelic compound where some of the heavy lifting of synthesis is accomplished by the biological expertise of the mushroom itself and not by conventional laboratory chemistry.
Jochen Gartz decided to attempt this by adding diethyltryptamine (DET, a close relative of DMT) to the fruiting body of psilocybe cubensis. He hoped that it would be hydroxylated to 4-HO-DET, or possibly phosphorylated even further to 4-PO-DET. He first colonized a mixture of cow dung and rice grain with psilocybe cubensis, and then injected it with a solution of DET. Within four weeks mushrooms were produced, and five total flushes of mushrooms were obtained.
| First | Second | Third | Fourth | Fifth | |
| 4-HO-DET | 2.5% | 0.2% | 3.1% | 3.3% | 2.1% |
| 4-PO-DET | - | 0.8% | 0.01% | - | 0.02% |
all values % by weight of dry mushroom
The project was a success, with significant amounts of 4-HO-DET produced. No DET was found in the dried mushrooms. A mass balance was not conducted to determine the efficiency of the conversion and possible losses in the fruiting body itself. The demonstrated non-selectivity of the enzymes in psilocybe cubensis toward other tryptamine derivatives opened to the door to the possibility of producing truly exotic and difficult to synthesize compounds such as 4-HO-5-MeO-DMT.
Despite this little additional data is available on the tryptamine derivatives that are able to be substituted in the fruiting body and the repeatability of the experiment. Some have found little success, noting only a decrease in the size of the mushrooms produced. Other attempts have discovered perhaps a qualitative difference in potency and character of the psychedelic experience, but this has not been substantiated by quantitative measurement.
Biotransformation of tryptamine derivatives in mycelial cultures of Psilocybe. Gartz, J. Journal of Basic Microbiology, Volume 29, Issue 6, Pages 347-352 (1989).