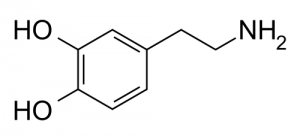
Norepinephrine
Norepinephrine (NE) is a neurotransmitter, involved in behaviours including alertness, anxiety, and attention. Most norepinephrine cells cluster in a pair of nuclei called the locus coeruleus, Latin for a “blue spot” in the brain stem. Each locus coeruleus is a long tube shaped cluster of norepinephrine neurons, both consisting of only 45,000 to 60,000 nerve cells in total. A second group of nerve cells arises deeper down in the medulla, and delivers norepinephrine mostly to the hypothalamus.
Primate brains have a dense network supplying norepinephrine to the posterior parietal lobe (which integrates sensory information), to part of the thalamus, and to the outer layers of the optic lobes in the midbrain. Relatively little norepinephrine is supplied to the temporal cortex, involved in speech, high-level visual processing (ie facial recognition), and memory. This outlines a distinctive feature of norepinephrine, that it is generally involved in direct sensory input rather than sensory processing. But the hypothalamus (which regulates hunger, thirst, fatigue, and sleep cycles) has the highest concentration of norepinephrine at 1150 nanograms per gram, indicating the vital role this neurotransmitter plays in deep primal instincts.
NE cells in the locus coeruleus fire especially in response to stressful, painful, noxious stimuli. In rats, they fire faster for up to thirty seconds if the toe is compressed in a painful manner. A strong but generic stimuli for cats (say a loud noise) will prompt a brief discharge of norepinephrine, but this response dwindles as the stimuli is repeated and the cat becomes conditioned.

In higher primates like monkeys, pain and stress also cause norepinephrine cells to fire faster. But what really gets monkey norepinephrine production going is fruit juice, their favorite treat. Norepinephrine cells fire at their highest rates when drinking fruit juice, seven to fifteen times per second. The norepinephrine cells of primate brains appear to be slightly different than that of lower animals, as they are especially activated by noxious stimuli and also certain specific attractive stimuli. Norepinephrine cells fire much slower if primates are given opiate based painkillers however, and during dream states (REM) of sleep.
So what exactly happens when norepinephrine cells fire more frequently? They appear to have rather few direct effects, but act primarily as a modulator. For instance, norepinephrine alone does little to change the slow background firing of cells in the hippocampus proper (involved in memory and spatial navigation) - but if these cells have been excited by addition of glutamate, norepinephrine makes them fire even faster. Norepinephrine also makes hippocampal cells fire faster if they have been excited by natural outside visual and auditory stimuli. It also reduces spontaneous background activity, increasing the signal to noise ratio of sensation from outside. This increased signal to noise ratio has become very valuable in treating symptoms of conditions such as ADHD. Norepinephrine and dopamine both play a large role in attention and focus. Various amphetamines and other compounds like methylphenidate (Ritalin) prescribed for ADHD cause higher levels of norepinephrine and dopamine.
Other compounds can act as agonists at norepinephrine receptors. Clonidine is used to relieve the suffering of addicts withdrawing from opiates. It activates certain autoreceptors which slow the norepinephrine cells’ firing rate, reducing symptoms such as anxiety, restlessness, and aching in muscles and joints. This clinical success emphasizes the theories linking excessive norepinephrine activity within the locus coeruleus with states of anxiety and tension. For instance, investigation into patients suffering panic attacks suggests that they may lack the normal mechanisms that would hold their norepinephrine systems in check.