Shulgin’s Sulfur Symphony - Part II
 Substitution of sulfur at the 4-position of 2,5-dimethoxyphenethylamine provides a building block for many successful psychedelic compounds, initially explored by Shulgin and named in the format 2C-T-x. Generally, smaller substitutions tend to produce compounds which act as agonists, while larger substitutions are partial agonists or antagonists. The smaller substitutions described in Part I tend to be potent psychedelics, while the larger substitutions discussed here trend toward stimulant effects or are inactive. Determining the precise boundaries of this relationship was a major motivation of Daniel Trachsel who continued Shulgin’s work with the larger substitutions of 2C-T-25 and above.
Substitution of sulfur at the 4-position of 2,5-dimethoxyphenethylamine provides a building block for many successful psychedelic compounds, initially explored by Shulgin and named in the format 2C-T-x. Generally, smaller substitutions tend to produce compounds which act as agonists, while larger substitutions are partial agonists or antagonists. The smaller substitutions described in Part I tend to be potent psychedelics, while the larger substitutions discussed here trend toward stimulant effects or are inactive. Determining the precise boundaries of this relationship was a major motivation of Daniel Trachsel who continued Shulgin’s work with the larger substitutions of 2C-T-25 and above.
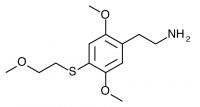 2C-T-13 (2,5-dimethoxy-4-(β-methoxyethylthio)phenethylamine) Active in doses from 25 to 40 mg, it produces a experience 6 to 8 hours in length. There is a focus on closed eye visual effects, with only slight visual distortions present if the eyes are open.
2C-T-13 (2,5-dimethoxy-4-(β-methoxyethylthio)phenethylamine) Active in doses from 25 to 40 mg, it produces a experience 6 to 8 hours in length. There is a focus on closed eye visual effects, with only slight visual distortions present if the eyes are open.
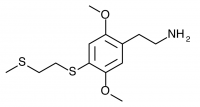 2C-T-14 (2,5-dimethoxy-4-(2-methylthioethylthio)phenethylamine) The sulfur counterpart to 2C-T-13. Synthesis has been taken to the nitrostyrene stage by Shulgin, producing “garish orange-red ‘Las Vegas’ colored crystals” which at the time of writing were “sitting on the shelf waiting to be reduced to the target compound”. It is unclear if the synthesis was completed, and no bioassays are publicly known.
2C-T-14 (2,5-dimethoxy-4-(2-methylthioethylthio)phenethylamine) The sulfur counterpart to 2C-T-13. Synthesis has been taken to the nitrostyrene stage by Shulgin, producing “garish orange-red ‘Las Vegas’ colored crystals” which at the time of writing were “sitting on the shelf waiting to be reduced to the target compound”. It is unclear if the synthesis was completed, and no bioassays are publicly known.
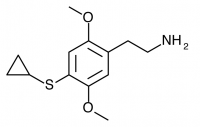 2C-T-15 (SESQUI, 2,5-dimethoxy-4-cyclopropylthiophenethylamine) Similar to 2C-T-8, with the cyclopropyl group one carbon closer to the phenyl ring. This compound appears to have been unremarkable, with only threshold effects noted at 30mg. Like 2C-T-8, this “particular substitution pattern is not one to set the world on fire”.
2C-T-15 (SESQUI, 2,5-dimethoxy-4-cyclopropylthiophenethylamine) Similar to 2C-T-8, with the cyclopropyl group one carbon closer to the phenyl ring. This compound appears to have been unremarkable, with only threshold effects noted at 30mg. Like 2C-T-8, this “particular substitution pattern is not one to set the world on fire”.
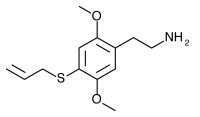 2C-T-16 (2,5-dimethoxy-4-allylthiophenethylamine) Synthesis was taken to the nitrostyrene stage by Shulgin, but has not been completed to public knowledge.
2C-T-16 (2,5-dimethoxy-4-allylthiophenethylamine) Synthesis was taken to the nitrostyrene stage by Shulgin, but has not been completed to public knowledge.
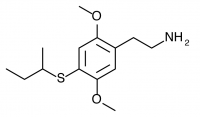 2C-T-17 (NIMITZ, 2,5-dimethoxy-4-sec-butylthiophenethylamine) Dubbed “Nimitz” by Shulgin after State Highway 17 from Oakland to San Jose (the Nimitz freeway), now called Interstate 880. Active in doses of 60 to 100 mg, it produces a 10-15 hour experience with alteration of thought patterns but little visual distortion. This compound is also notable for possessing a secondary butyl group containing an asymmetric carbon atom. Only racemic 2C-T-17 has been bioassayed, but Shulgin was extremely curious if the activity of the compound could be isolated to one of the two stereoisomers. This would be similar to the isolation of psychedelic effects to the R isomers of the substituted amphetamines, with their asymmetric carbon next to the amine group on the other side of the phenyl ring. Both stereoisomers of 2C-T-17 were brought to the nitrostyrene stage, but the independent synthesis of the individual stereoisomers was never completed to public knowledge.
2C-T-17 (NIMITZ, 2,5-dimethoxy-4-sec-butylthiophenethylamine) Dubbed “Nimitz” by Shulgin after State Highway 17 from Oakland to San Jose (the Nimitz freeway), now called Interstate 880. Active in doses of 60 to 100 mg, it produces a 10-15 hour experience with alteration of thought patterns but little visual distortion. This compound is also notable for possessing a secondary butyl group containing an asymmetric carbon atom. Only racemic 2C-T-17 has been bioassayed, but Shulgin was extremely curious if the activity of the compound could be isolated to one of the two stereoisomers. This would be similar to the isolation of psychedelic effects to the R isomers of the substituted amphetamines, with their asymmetric carbon next to the amine group on the other side of the phenyl ring. Both stereoisomers of 2C-T-17 were brought to the nitrostyrene stage, but the independent synthesis of the individual stereoisomers was never completed to public knowledge.
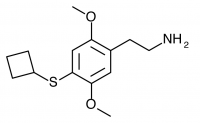 2C-T-18 (2,5-dimethoxy-4-cyclobutylphenethylamine) Synthesis was taken to the nitrostyrene stage by Shulgin, but has not been completed to public knowledge.
2C-T-18 (2,5-dimethoxy-4-cyclobutylphenethylamine) Synthesis was taken to the nitrostyrene stage by Shulgin, but has not been completed to public knowledge.
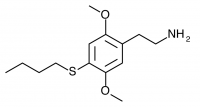 2C-T-19 (2,5-dimethoxy-4-n-butylthiophenethylamine) Synthesis was taken to the nitrostyrene stage by Shulgin, but not completed to public knowledge.
2C-T-19 (2,5-dimethoxy-4-n-butylthiophenethylamine) Synthesis was taken to the nitrostyrene stage by Shulgin, but not completed to public knowledge.
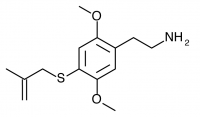 2C-T-20 (2C-T-3, 2,5-dimethoxy-4-(beta-methallyl)thiophenethylamine) Also known as 2C-T-3. Before working on the 2C-T series Shulgin investigated a similar series of promising compounds dubbed the Alephs, of which Aleph-3 was the beta-methallyl homologue. The synthesis of Aleph-3 was attempted, abandoned, and eventually forgotten. Years later the idea came to Shulgin again, and the beta-methallyl Aleph was begun anew along with the corresponding beta-methallyl 2C-T compound (2C-T-20). This led to the rediscovery of notes referencing the initial Aleph-3 synthesis attempt, and 2C-T-20 was renamed 2C-T-3 in order to maintain consistency with the Aleph project.
2C-T-20 (2C-T-3, 2,5-dimethoxy-4-(beta-methallyl)thiophenethylamine) Also known as 2C-T-3. Before working on the 2C-T series Shulgin investigated a similar series of promising compounds dubbed the Alephs, of which Aleph-3 was the beta-methallyl homologue. The synthesis of Aleph-3 was attempted, abandoned, and eventually forgotten. Years later the idea came to Shulgin again, and the beta-methallyl Aleph was begun anew along with the corresponding beta-methallyl 2C-T compound (2C-T-20). This led to the rediscovery of notes referencing the initial Aleph-3 synthesis attempt, and 2C-T-20 was renamed 2C-T-3 in order to maintain consistency with the Aleph project.
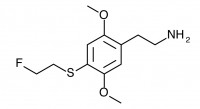 2C-T-21 (2,5-dimethoxy-4-(2-fluoroethylthio)phenethylamine
2C-T-21 (2,5-dimethoxy-4-(2-fluoroethylthio)phenethylamine
) The fluoroalkyl counterpart to 2C-T-7. Active in dosages between 8 and 12 mg it produces a 7 to 12 hour experience with a euphoric push. It was the first psychedelic compound synthesized which contained six separate elements, was widely regarded as a rich and unique material, and now languishes in obscurity due to an infamous incident that led to a large-scale DEA investigation.
On March 9, 2004, a 22-year-old quadriplegic man named James Edwards Downs in St. Francisville, Louisiana, consumed an unknown dose of 2C-T-21 by sticking his tongue into a vial of powder he had purchased online. He developed a high fever, had a tonic-clonic seizure, and slipped into a coma. Four days later, on March 13, Downs died at Lane Memorial Hospital in Zachary, LA.
This death became part of a two year DEA investigation called Operation Web Tryp which was launched in 2002. On July 22, 2004, the owners of American Chemical Supply were arrested on federal charges relating to distribution of controlled substance analogues and the death of James Edwards Downs.
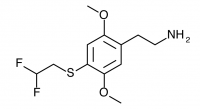 2C-T-21.5 (2,5-dimethoxy-4-(2,2-difluouroethylthio)phenethylamine
2C-T-21.5 (2,5-dimethoxy-4-(2,2-difluouroethylthio)phenethylamine
) Shulgin refers to this compound at the end of the 2C-T-21 entry in PiHKAL.
And it has just occurred to me that there is yet another effort that is certainly worth making, inspired by the observation that 2,2-difluoroethyl iodide is commercially available and not prohibitively expensive. It, with 2,5-dimethoxythiophenol, and following the obvious steps to the aldehyde, the nitrostyrene, and the final amine, would produce 2,5-dimethoxy-4-(2,2-difluoroethylthio)phenethylamine hydrochloride. It lies exactly half way between the highly potent 2C-T-21 (the mono-fluoro), and the yet to be finished 2C-T-22 (the trifluoro). Let’s be weird, and call it 2C-T-21.5. I will wager mucho that it will be very potent.
Synthesis of 2C-T-21.5 has not been completed to public knowledge.
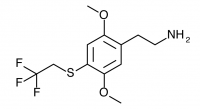 2C-T-22 (2,5-dimethoxy-4-(2,2,2-trifluouroethylthio)phenethylamine
2C-T-22 (2,5-dimethoxy-4-(2,2,2-trifluouroethylthio)phenethylamine
) Synthesis was abandoned due to difficulties in purifying the aldehyde, and has not been completed to public knowledge.
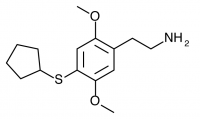 2C-T-23 (2,5-dimethoxy-4-cyclopentylthiophenethylamine
2C-T-23 (2,5-dimethoxy-4-cyclopentylthiophenethylamine
) Synthesis was taken to the aldehyde stage by Shulgin, but has not been completed to public knowledge.
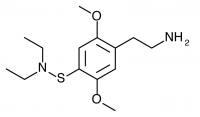 2C-T-24 (2,5-dimethoxy-4-diethylaminothiophenethylamine
2C-T-24 (2,5-dimethoxy-4-diethylaminothiophenethylamine
) Shulgin’s synthesis of this compound was unsuccessful, and it was not given a name. Murple dubbed it 2C-T-24. Shulgin describes his attempt in PiHKAL:
One additional effort was made to prepare a 2C-T-X thing with a sulfur-nitrogen bond. The acid chloride intermediate in the preparation of 2,5-dimethoxythiophenol (as described in the recipe for 2C-T-2) is 2,5-dimethoxybenzenesulfonyl chloride. It reacted smoothly with an excess of diethylamine to produce 2,5-dimethoxy-N,N-diethylbenzenesulfonamide which distilled at 155 °C at 0.13 mm/Hg and which could be recrystallized from a 4:1 mixture of cyclohexane/benzene to give a product with a melting point of 41-42 °C and an excellent proton NMR. This amide proved totally refractory to all efforts at reduction, so the target compound, 2,5-dimethoxy-4-diethylaminothiophenethylamine, has not been made. It has not even been given a 2C-T-X number.
This was the second attempt at creating a sulfur-nitrogen bonded phenethylamine, the first being 2C-T-12 which was also unsuccessful.
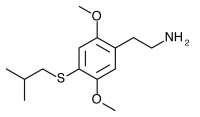 2C-T-25 (2,5-dimethoxy-4-isobutylthiophenethylamine
2C-T-25 (2,5-dimethoxy-4-isobutylthiophenethylamine
) The isobutyl to 2C-T-4’s isopropyl, or an unfluorinated 2C-T-21.5. This compound was synthesized by Daniel Trachsel but has not been bioassayed to public knowledge.
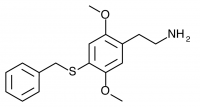 2C-T-27 (2,5-dimethoxy-4-benzylthiophenethylamine
2C-T-27 (2,5-dimethoxy-4-benzylthiophenethylamine
) Synthesized by Daniel Trachsel but has not been bioassayed to public knowledge.
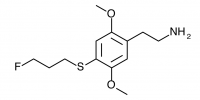 2C-T-28 (2,5-dimethoxy-4-(3-fluoropropylthio)phenethylamine
2C-T-28 (2,5-dimethoxy-4-(3-fluoropropylthio)phenethylamine
) The fluoroalkyl counterpart to 2C-T-19. Synthesized by Daniel Trachsel but has not been bioassayed to public knowledge.
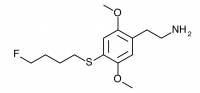 2C-T-30 (2,5-dimethoxy-4-(4-fluorobutylthio)phenethylamine
2C-T-30 (2,5-dimethoxy-4-(4-fluorobutylthio)phenethylamine
) 2C-T-28 with an additional carbon in the alkyl chain. Synthesized by Daniel Trachsel but has not been bioassayed to public knowledge.
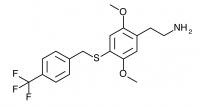 2C-T-31 (2,5-dimethoxy-4-(4-trifluoromethylbenzylthio)phenethylamine
2C-T-31 (2,5-dimethoxy-4-(4-trifluoromethylbenzylthio)phenethylamine
) A 4-trifluoromethyl substituted 2C-T-27. Synthesized by Daniel Trachsel but has not been bioassayed to public knowledge.
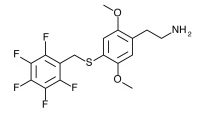 2C-T-32 (2,5-dimethoxy-4-(2,3,4,5,6-pentafluorobenzylthio)phenethylamine
2C-T-32 (2,5-dimethoxy-4-(2,3,4,5,6-pentafluorobenzylthio)phenethylamine
) A ring-fluorinated 2C-T-27. Synthesized by Daniel Trachsel but has not been bioassayed to public knowledge.
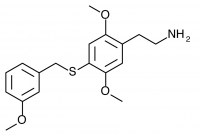 2C-T-33 (2,5-dimethoxy-4-(3-methoxybenzylthio)phenethylamine
2C-T-33 (2,5-dimethoxy-4-(3-methoxybenzylthio)phenethylamine
) A 3-methoxy substituted 2C-T-27. Synthesized by Daniel Trachsel but has not been bioassayed to public knowledge.
Trachsel, D. Synthesis of novel (phenylalkyl)amines for the investigation of structure-activity relationships. Part 2. 4-Thio-substituted [2-(2,5-dimethoxyphenyl)ethyl]amines (=2,5-dimethoxybenzeneethanamines). Helv. Chim. Acta, 5 Aug 2003, 86 (7), 2610–2619.


No comments yet.