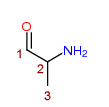
2-Aminopropan-1-one
Naphthylpyrovalerone (Naphyrone), one of many new cathinone analogues this amendment adds to Class B.
Effective 21 Jul 2010, S.I. 2010/1833 added:
1(ab)
Any compound structurally derived from 2-Aminopropan-1-one by substitution at the 1-position with any monocyclic, or fused-polycyclic ring system (not being a phenyl ring or alkylenedioxyphenyl ring system), whether or not the compound is further modified in any of the following ways, that is to say,
- by substitution in the ring system to any extent with alkyl, alkoxy, haloalkyl or halide substituents, whether or not further substituted in the ring system by one or more other univalent substituents;
- by substitution at the 3-position with an alkyl substituent;
- by substitution at the 2-amino nitrogen atom with alkyl or dialkyl groups, or by inclusion of the 2-amino nitrogen atom in a cyclic structure.
11 March 2016 ·  · Isomer Design
· Isomer Design
 · Isomer Design
· Isomer Design