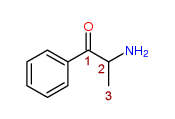
2-Amino-1-phenyl-1-propanone
Cathinone
Diethylpropion
Pyrovalerone
[Bupropion (above) is explicitly not captured by this clause]
4-Methylmethcathinone; Mephedrone, one of many new cathinone analogues this amendment adds to Class B.
Flephedrone, one of many new cathinone analogues this amendment adds to Class B.
Methylone, one of many new cathinone analogues this amendment adds to Class B.
Butylone, one of many new cathinone analogues this amendment adds to Class B.
MDPV, one of many new cathinone analogues this amendment adds to Class B.
Effective 16 Apr 2010, S.I. 2010/1207 added:
1(aa)
Any compound (not being Bupropion, Cathinone, Diethylpropion, Pyrovalerone or a compound for the time being specified in sub-paragraph (a) above) structurally derived from 2-Amino-1-phenyl-1-propanone by modification in any of the following ways, that is to say,
- by substitution in the phenyl ring to any extent with alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents, whether or not further substituted in the phenyl ring by one or more other univalent substituents;
- by substitution at the 3-position with an alkyl substituent;
- by substitution at the nitrogen atom with alkyl or dialkyl groups, or by inclusion of the nitrogen atom in a cyclic structure.
Only a handful of the Cathinone analogues captured by this amendment are shown below. For a more comprehensive enumeration, I highly recommend Synchronium’s elegant depiction, an inspiring model of information design.
CA: CDSA Schedule III Section 19 Cathinone
UN: Psychotropics Schedule I Cathinone
US: CSA Schedule I Section f Subsection 3 Cathinone
See also MDA Part III Class C Section 1(a) Cathinone
CA: CDSA Schedule IV Section 4 Diethylpropion
UN: Psychotropics Schedule IV Amfepramone (Diethylpropion)
US: CSA Schedule IV Section f Subsection 2 Diethylpropion
See also MDA Part III Class C Section 1(a) Diethylpropion
CA: CDSA Schedule IV Section 26 Pyrovalerone
UN: Psychotropics Schedule IV Pyrovalerone
US: CSA Schedule V Section d Subsection 1 Pyrovalerone
See also MDA Part III Class C Section 1(a) Pyrovalerone
EU: Substances Substances 4-Methylmethcathinone
US: CSA Schedule I Section d Subsection 36 4-Methylmethcathinone
⇒
PubChem: 45266826; ChemSpider: 21485694; Bluelight: Mephedrone; Drugs Forum: Mephedrone; Erowid: 4-Methylmethcathinone / Mephedrone; Wikipedia: MephedroneUS: CSA Schedule I Section h Subsection 17 4-Fluoro-N-methylcathinone
⇒
PubChem: 49853406; ChemSpider: 21477355; Drugs Forum: Flephedrone; Erowid: 4-Fluoromethcathinone (4-FMC); Wikipedia: 4-FluoromethcathinoneUS: CSA Schedule I Section d Subsection 47 3,4-Methylenedioxy-N-methylcathinone
US: CSA Schedule I Section h Subsection 14 Butylone
CA: CDSA Schedule I Section 17.1 Methylenedioxypyrovalerone
EU: Substances Substances 3,4-Methylenedioxypyrovalerone
US: CSA Schedule I Section d Subsection 37 3,4-Methylenedioxypyrovalerone
11 March 2016 ·  · Isomer Design
· Isomer Design
 · Isomer Design
· Isomer Design